* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chem*3560 Lecture 33: Membrane receptors and signalling
Protein mass spectrometry wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
Western blot wikipedia , lookup
Protein domain wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
List of types of proteins wikipedia , lookup
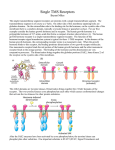
Chem*3560 Lecture 33: Membrane receptors and signalling Complex organisms indicate developmental or metabolic states by release of signalling molecules or hormones from one tissue to stimulate a response in another tissue. Some of these signalling molecules are relatively nonpolar and can pass through the bilayer. Steroids like estradiol or testosterone enter cells and bind to soluble receptor proteins in the cytoplasm. The complex is imported into the nucleus where the receptor acts to regulate gene expression. In animals, more polar signalling molecules such as epinephrine bind to specific receptors on the exterior of the plasma membrane. This is also the case for larger signalling molecules such as the peptide hormones insulin and glucagon. These receptors are transmembrane proteins, and presence of the ligand bound to the exterior is sensed by the cytoplasmic domain to pass the signal on to the cell interior. Insulin receptors, as well as the receptors for a variety of cell growth factors, are transmembrane proteins with a ligand binding domain on the extracellular side and a tyrosine kinase domain on the cytoplasmic side. Glucagon and epinephrine receptors have seven transmembrane helices, and regulate adenylate cyclase on the cytoplasmic side. The insulin receptor tyrosine kinase The insulin receptor tyrosine kinase consists of a dimeric structure with two α subunits containing the insulin-binding domains, and two β subunits with extracellular domain, a single transmembrane helix and a cytoplasmic tyrosine kinase domain, which terminates in an activation domain that contains tyrosine. When the receptor binds insulin, a reorientation of the cytoplasmic domains occurs and this allows activation by autophosphorylation of as many as three tyrosines near the C-terminal end of each β subunit. (More accurately, this is mutual cross-phosphorylation within the dimer) (Lehninger p.445). The unphosphorylated loop containing Tyr occupies the substrate site, but is constrained so that phosphorylation can't occur in the absence of insulin. Once activated, the catalytic site becomes available for the tyrosine kinase to phosphorylate other target proteins. Insulin has growth regulating and metabolic effects on cells Insulin behaves much like a variety of tissue specific growth factors, for example, fibroblast growth factor, epidermal growth factor etc. Each has a unique receptor so that a particular cell type only responds to its own growth factor. In many cases, the receptor is monomeric in the absence of ligand, and only forms dimer in the presence of ligand. Proximity due to dimer formation then allows the cross-phosphorylation of each others tyrosines. Once the receptor activates its intracellular tyrosine kinase, the growth stimulating signalling pathway inside the cell follows common elements. The insulin receptor phosphorylates a protein called insulin receptor substrate, IRS-1 at up to seven tyrosine sites. The phosphorylated IRS-1 binds to and stays closely associated with the insulin receptor phosphotyrosines. The signal that results from activated tyrosine kinase is often communicated through a series of protein-protein binding interactions. Animal cells contain a variety of proteins that contain related domains called SH domains which stands for Src Homology. These share sequences similar to domains in Src, one of the first tyrosine kinases to be discovered. (The name Src derives from its discovery in cells infected with the tumour-inducing chicken sarcoma virus.) SH1 is the tyrosine kinase catalytic domain itself. SH2 domains bind tightly to phosphotyrosine in another protein. SH3 domains bind a specific proline rich sequence found in certain intracellular signalling proteins. When IRS-1 becomes phosphorylated, a SH2-domain protein Grb2 binds to IRS-1's phosphotyrosines. Grb2 is an adapter protein whose function is to hold other proteins together. (This a role similar to TRADD, which links up tumor necrosis factor receptor to procaspase 8 in the apoptosis pathway). Grb2 also has two SH3 domains, which exist in a complex with a protein called Sos (Son of sevenless - don't ask why, it's too long a story). Because of IRS-1 is also bound to insulin receptor, these interactions concentrate Sos in proximity to the inside of the plasma membrane (Lehninger p.446). Here Sos can meet a lipid anchored protein called Ras (first found associated with Rat sarcoma virus). Since the insulin receptor signalling pathway regulates cell growth, it's not surprising that some of the factors are implicated with tumour induction. Ras is a GTP-activated signalling molecule that controls cell proliferation Ras is a member of a group of small regulatory GTPase proteins (230 amino acids) which are themselves regulated by binding GTP. Members of this family regulate a variety of cellular processes, e.g. Ran - nuclear transport; Rac - vesicle traffic, Rho - actin cytoskeleton. Ras binds GTP and hydrolyses it very slowly (turnover number 1.2 hr-1, which is 106 times slower than a typical enzyme). Ras passes on an "active" signal when it contains GTP, and an "inactive" signal when it contains GDP. The slow GTP hydrolysis serves as a timing mechanism, and determines how long Ras stays in its active state. Ras binds GDP very tightly so is unable to release the product, so remains in the "inactive" signalling state. Sos plays the role of a guanine nucleotide exchange factor or GEF. When Sos binds Ras, it allows Ras to exchange the tightly bound GDP for a new molecule of GTP, thus switching it from an inactive to active state. Finally Ras is the activating factor for a Ser-Thr protein kinase called Raf (Ras activated factor). Raf initiates a serine-threonine kinase cascade (MAP Kinase, mitogen associated protein kinase) which passes on into the nucleus and activates transcription factors, in particular for genes associeted with cell proliferation. In addition, Raf serves as a GTPase accelerating protein or GAP, and speeds up the GTPase rate of Ras to a respectable 20 s-1 (105 fold speed up). Before Raf binds, slow GTP hydrolysis keeps Ras active, giving it a good chance to meet up with Raf. Fast GAP-induced hydrolysis of GTP rapidly turns off the Ras "active" signal as soon as Ras has done its job. This combined effect of a specific GEF (guanine exchange factor) to turn on the signal and a GAP (GTPase accelerating protein) to turn it off again is a mechanism shared by many of the other small regulatory GTPases. Metabolic regulation by insulin receptor IRS-1 also stimulates another enzyme calle PI3K, phosphotidyl inositol-3 kinase, which adds another phosphate to the membrane phospholipid phosphatidyl inositol- 4,5-bisphosphate making it into phosphatidyl inositol-3,4,5-trisphosphate (PIP3 ). This serves as an activator of PIP3 -dependent protein kinase (PDK), which initiates protein kinase cascades that activate glycogen synthase and glucose transport (Lehninger p.447).