* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lewis Acid receptors
Survey
Document related concepts
Transcript
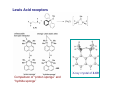
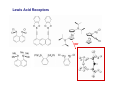
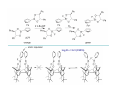
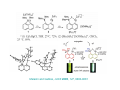
Lewis Acid receptors X-ray crystal of 4.69 Comparison of “proton sponge” and “hydride sponge” Lewis Acid Receptors OH- log K11 = 6.3 (CHCl3) Melaimi and Gabbai, JACS 2005, 127, 9680-9681 Sensing fluoride ion in water Kim and Gabbai, JACS, 2009, 131, 3363–3369 Increasing hydrophobicity R = Ph R = Me, K = 840 M-1 R = Et, K = 2500 M-1 R = n-Pr, K = 4000 M-1 R = Ph, K = 10,500 M-1 Measured in H2O/MeOH (9/1 vol.; 9 mM pyridine buffer, pH 4.6). Anticrowns A crown ether analogue made up from electron acceptor residues, as opposed to Lewis bases in the conventional crown ethers, can be thought of as an anticrown because of its opposite complexation behavior—affinity for Lewis basic anions instead of Lewis acids such as alkali metal cations. Chloride inclusion within the carborane-based mercura[12]crown-4 F- Cl- Metal Containing Receptors Anion receptors based on metal centers are a well established class of anion binding compound and can be classified into three broad categories: • Those in which an inert or labile metal centre plays a structural role • Those in which an inert or labile the metal is a key component of the anion binding site • Those in which an inert or labile metal acts as part of a redox, luminescent or colorimetric reporter or sensing group Use of an inert Ru(II) center to organize an anion chelate ligand Labile coordination compounds are not true anion hosts in the conventional sense. Instead, they fall into the category of self-assembly and are frequently templated by anions, cations or both. Self-assembly of a labile nitrate-binding Ag(I) complex and its evolution in the presence of excess nitrate. Organometallic Receptors Cyclic voltammetric waves (a) in the absence and (b) in the presence of Cl-. Br- Cyclic Voltammetry of Supramolecular Compounds (a) Potential versus time profile in a cyclic voltammetric experiment. (b) The resulting current versus potential trace (voltammogram) for a reversible redox process. ipa = current for anodic peak, ipc = cathodic (assuming scan to positive potential), E electrode potential, usually very close to the formal redox potential for the redox process under study. Schematic diagram of the experimental apparatus for a cyclic voltammetric experiment The working electrode is the electrode at which the electrochemical process being investigated takes place. The reference electrode is the electrode with a constant potential that is used as the reference standard against which the potentials of the other electrodes in the cell are measured. The auxiliary electrode serves as a sink for electrons so that current can be passed from the external circuit through the cell. Redox-switchable cation-binding cryptands Y3+ and Eu3+ binding lead to anodic shifts by 326 and 302 mV respectively, whereas Na+ binding results in only a 70 mV anodic shift. Venus flytrap anion sensor Cl- Simultaneous cation and anion receptors Schematic illustrating the various states that ions are generally found in solvents, i.e. (a) solvated ion pairs, (b) contact ion pairs and (c) aggregated contacted pairs. In real-world applications, non-competitive counter-ions are not generally encountered and hence inter-ion competition can be significant. Also, salts rarely, if ever, exist as separate ions unless the medium is highly solvating. Therefore, the most effective ionic recognition strategy is to design a receptor capable of explicitly recognizing both the anion and cation of an ion pair (either as a contaction pair or in different regions of the receptor) within the same molecular host. Schematics of three different types of simultaneous receptors Cascade receptor Ditopic receptor Zwitterion receptor • Cascade receptors When more than one metal ion (cation) coordinates to a particular ligand (often a Schiff base or macrocyclic heteroalkane) in a well-defined geometry and the anionic species then coordinates to the metal centre, this complex is known as a casacade complex. Dioxygen binding by haemocyanin • Ditopic receptors Ditopic ion-pair receptors involve the binding of ion pairs, either as contact or separated ion pairs with a separate binding site for the cation and another site for the anion. Alkali-metal chloride transport (symport) through a membrane • Zwitterion receptors Zwitterionic receptors differ from the two previous examples because the charges are both on the same molecule, hence rendering the receptor’s overall charge neutral. Chiral discrimination of amino acid derivatives by the zwitterions (+)-tubocurarine (2.113) A zwitterion duplex involved in templation of a condensation reaction between complementary components to give rudimentary self-replication. • Cation and neutral simultaneous receptors The X-ray structure and chemical diagram of the receptor 2.116, binding Na+ and a single molecule of toluene within the cavity.