* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energetics of the nerve terminal in relation to central nervous system
Lactate dehydrogenase wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Western blot wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biochemistry wikipedia , lookup
Brain Bioenergetics
Bioenergetics Group and Neurochemical Group Joint Colloquium Organized by G. Brown (University
College London) and C. Cooper (University College London Medical School), Edited by G. Brown
(University College London) and Sponsored by Merck Sharpe and Dohme, The Wellcome Foundation and
Hamamatsu Photonics UK Ltd. 65 I s t Meeting held at University of Lancaster, 13- I4 July I994
Energetics of the nerve terminal in relation to central nervous system function
Maria Erecinska*§, David Nelson*, Marc Yudkofft and Ian A. Silver$
*Department of Pharmacology and tkpartment of Pediatrics, University of Pennsylvania, School of Medicine and
Children's Hospital of Philadelphia, PA 19104, U.S.A. and $Department of Anatomy, School of Veterinary Science,
University of Bristol. Bristol 852 8EJ, U.K.
Introduction
The main function of the mammalian central
nervous system (CNS) is the generation, processing
and transmission of impulses all of which require
movements of ions down their concentration
gradients. T o perform these activities, the key
cations, Na+, K + and Ca2+,have to be maintained
in electrochemical disequilibrium across the plasma
membrane, a state which has to be rapidly reinstated before each functional cycle. Because maintenance of disequilibria requires the constant input
of energy, 5 0 4 0 % of ATP produced in the CNS
during rest, and 90% or more generated during
enhanced activity, is consumed to support ion
movements [ 11.
Brain contains ATP (2-3pmoVg) and ADP
(0.2-0.5 pmol/g) at an ATPIADP ratio of 8-10
and the additional energy reservoire, comprising
creatine phosphate and creatine, at a total concentration of 10 pmol/g and a creatine phosphate]
creatine ratio of 0.8-1.0 [l]. The key respiratory
substrate is glucose, and under physiological conditions, with oxygen consumption rates of
1.6-5.4 pmol/g (depending on the animal), very
little lactate is produced [l]. Thus brain relies
heavily on glucose and oxygen to support its energy
metabolism. These relationships are illustrated in
Table 1 , which summarizes events occurring in
hippocampal CA 1 neurons during short-term
(8 min) ischaemia and subsequent recovery. It is
Abbreviations used: CNS. central nervous system;
[Na I,, intracellular [Na 1; TCA, tricarboxylicacid.
$To whom correspondence should be addressed, at:
Department of Pharmacology. University of Pennsylvania School of Medicine. Philadelphia, PA 19 104-6084.
U.S.A.
+
+
clear from the figures presented that glucose is
exhausted very rapidly and that the lack of oxygen
leads to massive movements of ions, with consequent collapse of their gradients. Internal K + concentration decreases and Na+, CaL+,H+ and CIconcentrations increase. However, this situation is
swifily and almost completely reversed when
oxygen and glucose are reintroduced, which indicates that neurons have powerful mechanisms that
restore ion gradients and that aerobic ATP generation is able to support these processes.
Studies of the mechanisms which control
energy production and ion movements in whole
brain are dificult. This has led to the development
of several model systems in which conditions can
be more readily manipulated and controlled. One
such system is the preparation of nerve-ending
particles (synaptosomes) which possesses many
properties of intact neurons. This paper describes
energetic properties of synaptosomes and the interrelationships between their energy level and production and ion movements and gradients.
Energy level and production in
synaptosomes
Synaptosomes isolated from brains of nonanaesthetized animals and equilibrated with oxygen
and glucose for 10-15 min contain ATP (1.405.6 nmol/mg of protein) and ADP (0.4-2.45 nmol/
mg of protein) at a ratio of 1.4-6 (range of values
reported by different laboratories 2:l [2-81) and
phosphocreatine (2.2-5.8 nmol/mg of protein
[2,4,6- 101) and creatine ( 1 5-25 nmol/mg of protein
[7-lo]) at a ratio of 0.4-0.5. Creatine phosphokinase immunoreactivity is concentrated in nerve
terminals, at least in some regions of the brain [ 11
and brain mitochondria exhibit high activity of this
959
-
I994
Biochemical Society Transactions
Brain glucose levels, membrane potentials and intracellular ion concentrations in hippocampal CAI neurons
during short-term low-flow ischaemia and recovery
960
All parameters were measured with microelectrodes. External concentrations of the ions of interest are as follows (in mM): Na’,
133 f 2.2; K + . 3.37f0.05; Ca2+. 1.45f0.20 CI-. 1 3 5 f 4 and H+ as pH, 7.34f0.02. Data taken from [20] and provided by
M. Erecinska. D. Nelson, M. Yudkoff and I. A. Silver (unpublished work). Values are means f SD ( n = 12-104).
Control
lschaemia(8min)
Recovery ( I0 min)
2.4fO.l
-67.0f7.4
O.OfO.O
3.5 f 0.9
- 17.0f9.3
- 4 I .3 f 18.0
0.089f0.024
30.2f 11.3
0.354 f 0. I3 I
7.33f0.04
6.21 f0.45
6.94 f 0.22
25.5f2.3
72.3f9.6
33.7 f 6.4
83.6f3.9
37.1 f 11.3
76.8 f 8.2
24.7f5.8
67.2f8.1
38.7 f I I .7
Effect of enhanced ion movements on synaptosomal rates of energy generation
All measurements were carried out at 37°C. The rates of lactate production were calculated from the difference in the level of this
metabolite at ‘time zero’ (i.e. after 10 min of preincubation with glucose and Ca2’) and after 5 min of incubation either with (experimental) or without (control) the compounds indicated. Nucleotides were measured by h.p.1.c. on neutralized perchloric-acidextracts
of synaptosomes quenched after 5 min incubation. The rates of ATP production were calculated assuming stoichiometric factors of 6
(0, uptake) and I (lactate generation) respectively. Values are meansf S.E.M. ( n = 3-8).
Condition
0, uptake
Lactate production
(nmol/min
per mg of protein)
Control
Veratridine (40pM)
Veratridine (40 pM) +
ouabain (I mM)
Monensin ( I0 pM)
Nigericin (5 pM)
5.20 f 0.22
16. I0 f I .03
4.72 f 0. I I
I .40 f 0. I7
3.08 f 0.52
0.63 f 0.07
32.6
99.7
29.0
4
3
2
I .80 f 0.07
I. I3 f0.04
I .56 f 0.09
5.8 I f 0. I6
I .8 I f 0.09
2.95 f 0.32
8.58 f 0.78
18.2 f I .30
6.04 f I .08
69.7
73.2
26
8
0.72 f 0.05
0.43 f 0.07
I .2 I f 0.03
0.69 f 0.04
I I .20 f 0.44
enzyme [ l l ] . The rate of oxygen consumption
ranges from 2 to 4nmoVmg of protein per min at
25-30°C and from 5.2 to 11 nmol/mg of protein
per min at T C , and that for lactate production
from 0.3 to 0.5 and from 0.8 to 2.7 nmol/mg of protein per min respectively at the same temperatures
(Table 2) [ 3,6,8,12- 141. Thus oxidative phosphorylation provides over 90% of total ATP produced
under aerobic conditions (see Table 2 for calculations). A comparison of the values measured in synaptosomes with those in whole brain indicates that
both the content of the high-energy phosphate compounds and the glycolytic and mitochondria1 oxidative activity of the terminals are 5-10-fold lower
than those in the intact organ. It has been proposed
that this behaviour is a consequence of the heterogeneity of the preparation which, it was suggested,
was composed of vesicles with differing degree of
Volume 22
ATP
production
Glycolytic
contribution
(%)
ATP (nmol/
mg of protein) ATP/ADP
‘intactness’ and different content of mitochondria,
only some of which had high ATP/ADP ratios and
the capacity to produce energy [6]. However, this
conclusion was based on the finding that the overall
ATP/ADP was only 2.18, whereas values as high
as 5-6 have been obtained in studies by other
authors (Table 2) [4,15]. Moreover, Leong et al.
[ 161 reported that the activities of several enzymes
of the tricarboxylic acid (TCA) cycle in synaptic
mitochondria were at least 2-fold lower than in the
non-synaptic organelles, which suggests that the
intrinsic activity of the energy-producing pathways
in the nerve endings may be lower than in cell
bodies or glial cells. It should be remembered that
small but constant loss of ATP from the terminals
may also occur through exocytosis in the presence
of calcium in the medium and contribute to the low
nucleotide level.
Brain Bioenergetics
Although synaptosomes, in contrast to many
intact cells, are able to transport and oxidize several
intermediates of the TCA cycle [12], it is not clear
at what concentration these molecules are present
in vivo;thus glucose is likely to be the key physiological substrate. If this is true, two issues deserve
comments: the transfer of NADH from the cytosol
to the mitochondrion; and metabolism of pyruvate
by pyruvate dehydrogenase. With respect to the
first, it has been shown that inhibition of transamination either by amino-oxyacetate [17] or Bmethylene-aspartate [ 181 inhibits glucose oxidation
and lowers the synaptosomal ATPlADP ratio while
increasing lactate production and the lactatelpyruvate ratio. Because both treatments inhibit transamination reactions, which are a necessary component of the malate-aspartate shuttle, it has been
suggested that the latter plays an important role in
the transfer of reducing equivalents across the mitochondrial membrane. Interestingly, the same
phenomenon was observed in the presence of
3-nitropropionate. an inhibitor of succinate dehydrogenase and a mitochondria1 poison, which
lowers intrasynaptosomal aspartate level, most
probably by depletion of oxaloacetate [ 191.
With respect to the role of pyruvate dehydrogenase in synaptosomal energy production,
this enzyme is regulated by a number of factors,
one of which is Ca" [20]. It has been shown that
synaptosomal pyruvate dehydrogenase is present
largely (80-90%) in its active state and that an
increase in internal [Ca" ] increases this proportion
only slightly [21]. There is, moreover, some controversy with respect to the role of Ca" in synaptosoma1 pyruvate metabolism. Whereas Patel et al.
[22] were unable to observe any stimulation of
oxygen uptake by veratridine in the absence of
external Ca" and with pyruvate as the respiratory
substrate, no requirement for this cation under
apparently identical conditions was seen in studies
of other investigators [ 23,241. This suggests that
CaL+ may not be a major regulator of pyruvate
dehydrogenase in the nerve endings.
One of the interesting issues in brain energy
metabolism is to what extent other fuels can replace
glucose as the energy source. Bradford and coworkers [25] have shown that glutamine is a major
substrate for the nerve endings, and it is known that
during in vivo hypoglycemia, the levels of glutamine
and glutamate decrease and that of aspartate
increases. Using "N-labelled amino acids, it was
possible to demonstrate that transamination from
glutamate to aspartate is very active [26,27] and that
a series of reactions may operate in which gluta-
mate produced from glutamine through the glutaminase reaction is transaminated to aspartate with
the production of 2-oxoglutarate, which is then
oxidized in the TCA cycle with the synthesis of
ATP and regeneration of oxaloacetate. Consistent
with this suggestion are the results of experiments
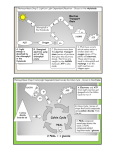
with deuterated glutamine (Figure la) (M. Yudkoff
D. Nelson, Y. Dai Khin and M. Erecinska, unpublished work) which show the rapid appearance of
labelled succinate, malate and aspartate. From the
extent and pattern of labelling, the rate of this segment of the TCA cycle in the presence of glucose
was calculated to be 3.14-6.65 nmollmin per mg of
protein (range of numbers derived from calculations from different labelled precursors) at 30°C.
Using a similar approach but another labelled precursor, [3-13C]aspartate (Figure lb), the rate of segment between malate and 2-oxoglutarate was
estimated to be somewhat smaller, 0.92-2.57 nmoll
min per mg of protein. In contrast to the high
activity of the aspartate transaminase reaction [27].
that of glutamate dehydrogenase is very slow [28].
which suggests that synaptosomes conserve
glutamate. It is possibly significant that two endogenous constituents of the nerve cell, M&+ and
polyamines, are powerful inhibitors of brain
glutamate dehydrogenase [29].
Synaptosomal ion concentrations
Synaptosomes contain potassium at a concentration
of 45-65mM [10,13,30,31] and maintain a K + diffusion potential equivalent to - 50 to - 60 mV; this
agrees well with estimates of the membrane potential from the distribution of XhRb [32]. Values
derived from the distribution of the radioactive lipophylic cations are somewhat higher [33], most
probably because of binding (andlor sequestration)
of these probes to synaptosomal constituents.
Measurements of intracellular [Na+1, "a'],,
in synaptosomes (e.g. atomic absorption or distribution of zzNa) at physiological levels of the latter are
more difficult to make because of the large contamination with the external fluid containing high
concentrations of the cation. Hence, not surprisingly, the original calculations yielded figures for
"a+], upwards of 50mM [13]. However, if the
internal and trapped volume are measured simultaneously and the necessary corrections then made,
estimated values range from 25 to 29mM at
130-140 mM NaCl in the medium [34,35], which is
not much different from [Na'], in neurons in vivo.
Recently, "a'],
was determined from the fluorescence of an indicator, benzofuran isophthalate,
I994
96 I
Biochemical Society Transactions
~~
Diagram outlining pathways of tracers used to measure flux through the
TCA cycle between 2-oxoglutarate and oxaloacetate (a) and oxaloacetate
and 2-oxoglutarate (b)
962
In (0)~-[2,3,3,4,4-'H]glutaminewas used, and in (b). 1-[3-'~C]aspartate.
A denotes 'H,
identifies ' 'C.
cytosol
*
Mitochondrion
(4
Glucose+Pyruvme
\
AGlutamine
A Glutamine
lsoatrate
I
~ASUCClnnto
8
*Aspartate
and reported to be 10.9 mM [ 361, i.e. considerably
less than the figures above. Whether this disparity
can be accounted for by the difference in methodology or whether the concentration of sodium in the
nerve endings is indeed lower in the nerve cell
body, cannot be decided at present.
The advent of fluorescent indicators for CaL'
has enabled estimates to be made of the free internal concentration of this cation in structures which
are too small to be penetrated with microelectrodes.
Although the number of studies using either Quin-2
or Fura-2 is very large, they do not differ significantly from the original estimates of Ashley et al.
[ 371, Richards et al. [ 381 and Hansford and Castro
[21]. The figures fall between 0.1 and 0.35 ,uM and
depend to some extent on the state and/or quality
Volume 22
'Oxaloacetate
f
\\
-+*Malate
A
\
'cisAconitate
I
1
\
\
\
'.
Fumarate
R
'2sxoglutarate
/
/
-SuccinateY
of the synaptosomal preparation (see [ 391 and references therein).
There are three other ions of interest for
which some results are available. Measurements of
intrasynaptosomal pH indicates that the concentration of protons inside the nerve ending is slightly
higher than that in the external environment, with
pH, values of 7.1-7.3 [ 14,38,40]. [Mg"], estimated
with an entrapped indicator eriochrome blue was
0.3 mM at 1 mM external [MgL'] and no CaL+.
With 1 mM CaL+ in the medium, the apparent
[Mg"], declined to 0.2mM, and at 2mM to
0.1 mM. The concentration of free chloride calculated from changes in fluorescence of N-(6-methoxyquinolyl) acetoacetyl ester in synaptoneurosomes from rat brain was found to be 14 mM [41].
Brain Bioenergetics
Relationships between ions and energy
metabolism
The key enzyme responsible for the maintenance of
ion gradients in the brain is the ouabain-sensitive
N a + / K + pump. This protein extrudes 3equiv. of
Na' and accumulates 2equiv. of K' with concomitant hydrolysis of one ATP. The estimated
maximal activity of this ATPase in synaptosomes is
l60-200nmol/min per mg or protein at 37°C in a
frozen-thawed preparation [ 421 and 260 nmol/min
per mg in the membrane fraction [43]. Ultrastructural localization studies on whole brain confirm
these in vitro results, in that they show intense
ATPase-specific staining over the entire plasma
membrane of the synaptic area [44]. Interestingly,
these same areas are very reactive for cytochrome
oxidase [45], which indicates that the maintenance
and restitution of ionic balances are energetically
costly.
The K,,, of the Na'/K+ pump for K' in
broken synaptosomes is low, 0.65 mM [42], which
agrees well with the ECs,, values for the K + dependent stimulation of lactate generation and
oxygen consumption (0.7- 1.5 mM) [24]. The K,,,
for Na+ is considerably higher than that for K + ,
and values reported in the literature range from 10
1421 to 80mM 1431. Hrodsky and Guidotti [46]
noted that Na' affinity of brain Na'/K'-ATPase
was dependent on both isozyme and environment
of the pump, the apparent dissociation constant
being much greater in synaptosomes than in their
membranes. There are two binding sites for ATP
on the pump, with differing affinities: the K,,, on the
catalytic site is low ( 10pM)whereas that on the
regulatory site is much higher, > 0.5 mM [I]. It is
evident from a comparison of the affinities for the
three substrates that, under physiological conditions, the key factor bhich regulates the pump
activity is [Na'],. llowever, the rather high K,,, for
ATP on the regulatory site indicates that changes in
concentration of the nucleotide may also be a contributory factor. particularly in some parts of the
neuron, such as the synapse, where the levels of
high-energy phosphate compounds may be lower.
Direct measurements of the ouabain-sensitive ""Rb
influx in the presence of amytal (an inhibitor of the
respiratory chain), either with or without glucose
1.241. confirm this supposition and show that a
decrease in pump activity can occur at an early
stage of limitation in ATP generation.
In non-stimulated synaptosomes incubated
with 5 mM K', the rate of ""Rb influx is 9.8 nmoV
min per mg of protein at 30°C: [24]. Addition of
ouabain under the same conditions decreases the
-
rate of oxygen uptake by 0.89 nmol/min per mg of
protein, which gives an Rb/O, ratio of 11.5 and a
Rb/ATP stoichiometry of 2, in agreement with the
known properties of the pump. Stimulation of pump
activity markedly increases the rate of K' uptake
[ 3 11 and simultaneously raises the rate of synaptosoma1 energy synthesis (Table 2) [ 14,24,47].
Increase in [Na'li, such as occurs after opening by
veratridine of the voltage-dependent Na -channels,
stimulates oxygen consumption by 2-5-fold
[ 3,6,8,24]. This rise is completely prevented by
addition of ouabain (Table 2), which indicates that
enhanced ion movements consume under such
conditions the overwhelming proportion of the
ATP produced. Glycolysis, albeit activated, contributes only marginally to total energy generation
(Table 2). Interestingly, when the activity of
phosphofructokinase (the rate-controlling enzyme
of glycolysis) is independently stimulated by an
increase in intrasynaptosomal pH caused by addition of the ionophore monensin (which exchanges
Na+ for H+), the glycolytic contribution to overall
ATP synthesis becomes much greater (Table 2)
[ 141. The role of glycolysis in supporting the N a + /
K+-ATPase is crucial when oxygen becomes limiting and the rate of oxidative phosphorylation
declines. This is underscored by experiments which
show that the rate of anaerobic K + emux, and
hence its loss from synaptosomes, is > 2-fold faster
in the absence of glucose [31]. Although there is
some evidence [20] that the pump uses the ATP
produced by glycolysis in preference to that
supplied by oxidative phosphorylation. when the
nucleotide level falls beyond a critical value, high
rates of lactate generation become insensitive to the
action of ouabain 1241.
In addition to constant uphill movements of
Na' and K'. synaptosomes also maintain a large
electrochemical gradient for Ca2+. There are two
mechanisms that expel CaL+ from the nerve endings: the Ca" pump and the Na'/Ca'+ exchanger
1481. The former is fueled directly by ATP and
exchanges CaL+ for 1-2 11'. The latter removes
one CaL' ion from inside against three Na' ions
entering from outside; as three Ka+ ions are
pumped out by the Na+/K+-ATPase per each
ATP hydrolysed, the energetic cost of the coupled
process is ICa'+ per ATP. The pump functions
predominantly under low Ca" loads, whereas the
exchange predominates at higher loads [ 491. The
rate of Ca2+ influx under non-stimulated conditions
is 0.5-10 nmol/min per mg of protein at 30-37°C
[SO], which corresponds to the same rate of ATP
utilization, or 0.1-0.2 nmol OL consumed/min per
+
I994
963
Biochemical Society Transactions
964
mg of protein. This amount is too small to be
detected experimentally. Upon membrane depolarization and opening of the voltage-dependent channels, Caz+ entry pathways can be activated by as
much as 3-&fold [SO], which would increase the
rate of energy utilization to 3-6nmoVmin per mg
of protein. However, the maximal increase in the
oxygen consumption which this would cause is
only
1 nmol/min per mg of protein, which is
small compared with that caused by movements of
ions through the Na+ pump. This may explain why
a rise in [Caz+],induced by administration of veratridine, monensin or nigericin has little effect on
synaptosomal respiration [ 14,24,47].
-
Concluding remarks
Like any model system, the synaptosomal preparation has advantages and pitfalls. Nevertheless, when
care is taken to purify vesicles with a high ATP/
ADP ratio, several properties of neurons can be
conveniently studied. Those discussed herein
include ion levels, movements and gradients and
the relationships between these parameters and
energy expenditure. A similarity between the results
obtained in this preparation in vitro and those in
brain in vivo makes it a valid model system for the
study of nerve-cell metabolism.
The authors’ research cited in this review was supported
by grants NS28329 and NS27889 from the National
Institutes of Health (LISA.).
1 Erecinska, M. and Silver, I. A. (1989) J. Cereb. Hlood
Flow Metab. 9,2-10
2 1)e Helleroche. J. S. and Bradford. H. F. (1972) J.
Neurochem. 19,585-602
3 Harvey, S. A. K., Hooth. H. F. G. and Clark, J. H.
(1983) Hiochem. J. 212,289-295
4 Kauppinen, H. A,. McMahon, H. and Nicholls. I). <;.
( 1988) Neuroscience 27. 175- 182
5 Kauppinen, K.A. and Nicholls, 1). G. ( 1 986)J. Neurochem. 47. 1804- 1869
6 Kyriazi, 11. T. and Hasford, H. E. (1980) J. Neurochem. 47.5 12-528
7 Hafalowska, U., Erecinska, M. and Wilson, 1). F.
( 1980)J. Neurochem. 34,1180- 1 186
8 Scott, 1. A. and Nicholls. L). G. (1980) Hiochem. 186,
21-33
9 Harvey, S. A. K.. Hooth, H. F. G. and Clark, J. H.
(1982) Hiochem. J. 206.433-439
10 Dagani, F. and Erecinska, M. (1987) J. Neurochem.
49, 1229- 1240
11 Jacobs, H., Heldt, H. W. and Klingenberg, M. (1904)
Hiochem. Hiophys. Hes. Commun. 16.5 16-52 1
12 Hafalowska, U.. Erecinska. M. and Wilson, 1). F.
( 1980)J. Neurochem. 34,1160- 1 165
13 Bradford, H. F.(1970) Hrain Hes. 19,239-247
Volume 22
14 Erecinska, M.. Dagani. F.,Nelson, D., Deas, J. and
Silver, I. A. (199 1) J. Neurosci. 11.24 10-242 1
15 Kauppinen. H. A. and Nicholls, 1). G. (1986) Eur. J.
Hiochem. 158,159- 165
16 Leong. S. F.. Lai. J. C. K.. Lim, 1,. and Clark. J. H.
(1984) J. Neurochem. 42.1306- 13 12
17 Kauppinen. K. A., Sihra, T. S. and Nicholls. L). G.
(1987) Hiochim. Hiophys. Acta 930, 173-178
18 Cheeseman, A. J. and Clark. J. H. (1988) J. Neurochem. 50, 1559- 1505
19 Erecinska, M. and Nelson. 1). (1994) J. Neurochem.
63, 1033- 1041
20 Erecinska, M. and Silver, 1. A. (1994) h o g . Neurobiol.
43,3741
21 Hansford, K. G. and Castro, F. (1985) Hiochem. J.
227. 129- 1 30
22 I’atel. T. H., Sambasivarao, L). and Hashed, H. M.
(1988) Arch. Hiochem. Hiophys. 264. 308-375
23 Kauppinen, K. A. and Nicholls. L). G. (1986) FEHS
1,ett. 199,222-220
24 Erecinska, M. and Dagani, F. (1990) J. Gen. Physiol.
95. 59 1-6 16
25 Bradford, 14. F.,Ward, H. K. and Thomas, A. J. (1978)
J. Neurochem. 30. 1453-1459
26 Erecinska. M., Zaleska. M. M., Nissim, I., Nelson. 11..
Dagani, F. and Yudkoff, M. (1988) J. Neurochem. 51,
892-902
27 Erecinska. M., Pleasure, D.,Nelson, D.,Nissim, 1. and
Yudkoff, M. (1993) J. Neurochem. 60. 1696-1706
28 Yudkoff, M.. Nissim, I.. Nelson, D.,Lin. Z. I’. and
Erecinska. M. (199 1) J. Neurochem. 57.60
29 Kuo, N.. Michalik. M. and Erecinska. M. (1994) J.
Neurochem. 63,
30 Hlaustein. M. 1’. and Goldring. J. M. (1 975) J. I’hysiol.
(London) 247.589-615
3 1 I’astuszko, A., Wilson, 1). F..Erecinska, M. and Silver,
1. A. (198 1 ) J. Neurochem. 36. 1 16- 123
32 Akerman, K. E. 0. and Nicholls, I). G. (1981) Eur. J.
Hiochem. 115,67-73
33 Deutsch, C. J. and Hafalowska, [I. (1979) FEHS 1,ett.
108,274-278
34 Akerman. K. E. 0. and Nicholls. 1). G. (1981) Eur. J.
Hiochem. 117,491-497
35 Erecinska, M.. Troeger. M. H. and Alston, T. A.
( 1986)J. Neurochem. 46,1452-1 457
36 1)eri. Z.and Adam-Vizi, V. (1993) J. Neurochem. 61,
8 18-825
37 Ashley, H. H.. Hrammer. M. J. and Marchbanks. H.
(1984) Hiochem. J. 219,149-158
38 Hichards. C. 11.. Metcalfe. J. C.. Smith. G. A. and
Hesketh, T. H. (1984) Hiochim. Hiophys. Acta 803,
2 15-220
39 Verhage. M., Hesselsen. E.. Lopes L)a Silva. F.H. and
Ghijsen, W. E. J. M. (1988) J. Neurochem. 51.
1667- 1674
40 Hoakye, I).. White, E. J. and Clark, J. H. (1991) J.
Neurochem. 57.88-94
41 Engblom. A. C. and Akerman. K. E. 0. (1993)
Hiochim. Hiophys. Acta 1153,262-266
Brain Bioenergetics
42 Kimelberg, H. K., Hiddlecome, S., Narumi, S. and
Hourke, R. S. (1978) Brain Res. 141,305-323
43 I,ogan. J. C. (1980) J. Neurocheni. 35.349-353
44 Stahl. W. I,. and Hroderson, S. H. (1976) Fed. I’roc.
35. 1260- 1265
45 Wong-Riley, M. T. T. (1989) Trends Neurosci. 12.
94- 101
46 Hrodsky. J. I,. and Cuidotti. <;. (1990) Am. J. I’hysiol.
258. C803-CX11
47 Erecinska, M., Nelson, 11.. Dagani. F.$Deas. J. and
Silver. 1. A. (1993) J. Neurochem. 61, 1356- 1368
48 Hlaustein, M. P. (1988) Trends Neurosci. 11,438-443
49 Sanchez-Armass, S. and Hlaustein, M. 1’. (1987) Am.
J. I’hysiol. 252, C595-Ch03
50 Hlaustein, M. P. (1975) J. I’hysiol. (Idondon) 247.
617-655
Received 20 May 1994
From the synaptosome to the intact brain
Risto A. Kauppinen
NMR Research Group, Department of Biochemistry and Biotechnology,A. I. Virtanen Institute, University of
Kuopio, P.O. Box 1627, FIN-70620 Kuopio, Finland
Introduction
Ikain function is centered at the synapse and, consequently, substantial scientific efforts have been
directed towards exploring synaptic function. In
neurochemical research, it has been customary to
divide neuronal parts of the synapse into two. Since
the early 1960s. ‘pinched-off presynaptic nerve
terminals or ‘synaptosomes’ have been extensively
used as a model of the presynaptic neuron [ 11. Our
present understanding of nerve-terminal metabolism and bioenergetics [2], neurotransmitter release
and uptake [ 3 ] , and electrophysiology is largely
based on studies carried on synaptosomes.
One of the interesting issues within neuroscience is the role(s) of 1.-glutamic acid. As the most
abundant cerebral transmitter, glutamate mediates a
majority of fast excitatory impulses in the cerebral
cortex. and is therefore strongly involved in integrated brain function [4].On the other hand, activation of postsynaptic glutamate receptors [ 51 evidently causes neuronal degeneration during brain
energy failure, for example, following ischaemia
[6,7]. In this paper, mechanisms of glutamate
release from synaptosomes are discussed with
major emphasis on their energy and CaL’ dependency and the contribution of various intraterminal
glutamate pools to this release [XI. This discussion
will be extended towards compartmentation of the
transmitter glutamate in the intact cerebral cortex in
the light of previous studies using ‘ € I and ‘€I{’.’C}n.m.r. spectroscopy [g-ll]. The aim in the latter
part will be to weight the relevance of synaptosome
studies to the conditions in an intact brain preparaAbbreviations used: [Ca’+I,. intracellular ICa” J; CI’MG.
Carr-l’urcell-Meiboom-Gill; NAA, N-acetylaspartate.
tion with special reference to the origin of ‘excitotoxic’ glutamate during severe energy failure.
Bioenergetics of synaptosomes
Virtually all ( > 90%) of the synaptosomes metabolizing glucose isolated from guinea-pig cerebral
cortex contain functioning mitochondria [ 121.
IJnstimulated nerve terminals respire in the absence
of glucose or in the presence of a glycolytic inhibitor, such as iodoacetate, at rates which are not different from those determined in the presence of
glucose. Oxidation of endogenous non-glucose substrates is, however, inefficient in maintaining the
ATPIADP ratio, which is reduced by 50% in the
absence of glucose [ 121. ‘Resting’ plasma or mitochondrial membrane potentials are not affected by
iodoacetate [ 121. Interestingly enough, it has been
reported that ATP levels in primary culture of cortical astrocytes are strictly supported through aerobic
glucose metabolism, with minor contribution from
mitochondria1 synthesis [ 131. In the absence of
glucose, astrocytic ATP levels and plasma-membrane potential collapse precipitously. Processes of
astrocytes reside perisynaptically and contribute
both to the maintenance of ionic and transmitter
homeostasis in the extracellular space [ 141. Assuming that a similar type of glucose dependency prevails in the brain in &YO, shortage of exogenous
glucose would primarily affect energy levels in
astrocytes. On the other hand, astrocytes contain
the majority of cerebral glycogen [ 1.51.
Synaptosomal energy state is highly sensitive
to ‘ischaemia-like’ conditions similar to the intact
brain [ 161. When oxidative phosphorylation is inhibited, either by a protonophore or by blocking
cytochrome oxidase, a precipitous drop of ATP and
I994
965