* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download a PDF of this article
Infection control wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Plant disease resistance wikipedia , lookup
Herd immunity wikipedia , lookup
Vaccination wikipedia , lookup
Gluten immunochemistry wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Complement system wikipedia , lookup
Social immunity wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Molecular mimicry wikipedia , lookup
DNA vaccination wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Autoimmunity wikipedia , lookup
Immune system wikipedia , lookup
Drosophila melanogaster wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Adaptive immune system wikipedia , lookup
Innate immune system wikipedia , lookup
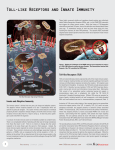
Innate Immunity Depends on Toll-Like Receptors From flies to mammals, these proteins provide a first-line defense and are implicated in infectious and autoimmune diseases William Check omething about the invertebrate Toll Center in Dallas and now at the Scripps Resystem and the mammalian toll-like search Institute in La Jolla, Calif., and his colreceptors (TLRs) inspires scientific laborators found similar receptors in mice. Early insight and intellectual excitement. Colon, they identified a receptor, designated TLR4, leagues recount how biologist Christhat specifically recognizes and binds lipopolytiane Nusslein-Volhard exclaimed “toll”— saccharides (LPS) from gram-negative bacteria. German slang, meaning “fantastic”—while Subsequently, researchers from several laboramarveling in 1980 over a Drosophila mutant tories revealed a family of (now) 10 mammalian that proved fruitful for explaining fly embryoTLRs that appear to be involved exclusively in genesis. The name stuck with that the innate immune response but mutant, and her efforts after that not in development. “Eureka” moment led NussleinSuch TLRs are “simply put, the Efforts to Volhard to a Nobel prize in 1995, main way that we [mammals] see understand the says Jean-Marc Reichhart of the the microbial world,” Beutler says. critical place of Louis Pasteur University in Stras“They are the initial gateway to TLRs in bourg, France. all— or nearly all—mammalian remammalian The prototypic Toll-1 receptor sponses to microbes.” TLRs can host defense identified by Nusslein-Volhard activate innate defenses, mainly inand another eight Toll proteins flammation, before adaptive imare giving rise serve developmental functions in mune responses come into play. to hopes for fruit flies. Additionally, Toll-1 Realizing that the TLR4 gene novel plays an important role in host despecifically recognizes LPS was “a therapeutic fenses against microorganisms, acshocking finding,” Beutler recalls. applications. cording to Reichhart and his colA mere few years ago, he and other leagues, including Jules Hoffman. biologists thought that mouse “In invertebrates, Toll is one of the TLRs served developmental funcmajor systems for protection and the first line of tions. Moreover, he says, “This finding told us defense against pathogens,” Reichhart says. that in mice a Toll homolog is required for “This is fascinating. How could these two funcresponse to gram-negative infection, while in tions have evolved under different pressures at flies Toll-1 is required for response to fungal two stages of fly life? Which was first? These are infection. We knew that this was more than a difficult questions to answer.” coincidence.” Other properties of the mouse from which the mutant TLR4 gene was cloned suggested that TLR4 senses LPS and mediates Toll-Like Components Important for host responses to this bacterial endotoxin. Innate Immunity in Mammals “That was really electrifying,” he exclaims. “I In 1998, immunologist Bruce Beutler, then at think it was the single most exciting moment of the University of Texas Southwestern Medical my scientific career.” S William Check is a science writer in Wilmette, Ill. Volume 70, Number 7, 2004 / ASM News Y 317 Continuing efforts to understand the critical place of TLRs in mammalian host defense are giving rise to hopes for novel therapeutic applications. “I think the possibilities are very large,” says Beutler. “And they will be even larger if it can be demonstrated that endogenous types of inflammatory disease, such as Crohn’s disease or ankylosing spondylitis, that involve overproduction of tumor necrosis factor-alpha (TNF-␣) are mediated primarily by activation of TLR pathways—although this is not known yet.” “TLRs will become important targets for pharmaceutical development,” agrees David Persing, vice president of Discovery Research at Corixa Corporation, Seattle, Wash. “They are relatively new targets in terms of recognition of their potential importance, and are involved in diseases ranging from infections to inflammatory diseases to autoimmune diseases and allergic disorders.” While scientists have been studying the adaptive immune response for several decades, their appreciation of the importance of innate immunity took shape only during the past few years, Persing says. “The innate response can in many ways dictate the tenor of the adaptive response.” Early Progress in Search for Simple Host Defenses in Studies of Fruit Flies Contemporary investigators siezed on the centrality of Toll-1 and TLRs to host defense in part because of efforts by their predecessors to answer an age-old question, one that is implicit in a verse by 18th-century Irish cleric and satirist Jonathan Swift. About the time that Antonie van Leeuwenhoek was peering through his microscopes to see “very little living animalcules,” Swift wrote: So, naturalists observe, a flea Hath smaller fleas that on him prey; And these have smaller still to bite ’em And so proceed ad infinitum. Swift humorously framed a simple fact—pathogens are ubiquitous. But how do organisms recognize and combat infections? When flies are infected by fungi, they effectively dose themselves with an antimicrobial metabolite, called drosomycin. But flies do not have diagnostic capabilities for determining whether they are infected and when to produce 318 Y ASM News / Volume 70, Number 7, 2004 internal antimicrobial defenses. How does that happen? And is anything similar about pathogen response mechanisms among other species? Similarities between Toll-1 and TLRs establish that link, and they are part of a more complex system for discriminating nonself from self. Indeed, the innate immune system uses genetically encoded receptors to respond to conserved microbial structures. Toll-1 in flies and the 10 human TLRs alert their respective hosts to infections. Reichhart and Hoffman recognized the antimicrobial function of Toll-1 while searching for the control mechanisms of innate immunity in Drosophila. “We asked very simple questions at the beginning,” Reichhart says. “When you challenge the insect with a microbe, it responds by expressing antimicrobial peptides. So what are those peptides?” They isolated the peptides, then the genes encoding the peptides. Next they asked, what is important about the promoters of the peptide genes? Response elements in these genes resemble those in mammals that bind the transcription factor, called nuclear factor- B (NFB), following activation by interleukin-1 (IL-1). A sequence search turned up only one NFB-like transcription factor in Drosophila, a protein called dorsal that is involved in early embryogenesis. A whole pathway was already known that triggers dorsal activity in the embryo. “The central key molecule in this signaling pathway that leads to dorsal is the transmembrane receptor Toll,” Reichhart says. In 1996 he and Hoffman and their colleagues reported that “mutations in the Toll signaling pathway dramatically reduce survival after fungal infection.” They later showed that Toll also is involved in sensing gram-positive bacteria. Comparable but More Complex Components in Mammals When Beutler in Dallas set out to find the mammalian gene responsible for sensing LPS, he soon focused on two mutant mouse strains. “It was known since 1965 that these mice were unable to survive challenge with gram-negative bacteria, but that their responses to things other than endotoxin were normal,” he explains. By positional mapping, he and his collaborators identified a critical region in which the best candidate FIGURE 1 The TLRs (of which there are 10 in humans) signal via interactions with TIR adapter proteins, of which five are known. Four are shown here; the function of the fifth (SARM) is not known yet. TLR4 (the LPS receptor) uses all four TIR adapters. TLR2 uses two; TLRs 3 and 9 each use one. It is likely that they are dimeric as shown. The usual pathway used is via IRAK4/IRAK, TRAF6, TAK1, and numerous other kinases, to activate NF-B. But Trif/Trif and Trif/Tram act to activate IRF-3 (Trif can also activate TRAF6). This is the basis of so-called “MyD88-independent signaling.” Note that TLR3 has no requirement for MyD88. In a manner not yet understood, TLR9 can also activate synthesis of type I interferons. Hence both TLRs 3 and 9 are very important for antiviral defense. MyD88 and Trif adapters are as well. It is at the adapter level that antiviral and antibacterial pathways converge. was the gene encoding mouse TLR4. Five other mouse genes that are also similar to TLR had been identified in EST databases. “However, no one knew what any of them did until our work was published,” Beutler says. “The fact that TLR4 sensed LPS immediately suggested that other TLRs might sense other molecules unique to microbes.” The following year, Shizuo Akira of Osaka University and his collaborators reported that TLR2 knockout mice are hyporesponsive to gram-positive bacterial cell walls, including to peptidoglycan from Staphylococcus aureus. Molecular recognition functions have since been assigned to other TLRs, including TLR1 to lipopeptide; TLR3 to double-stranded RNA (dsRNA); TLR5 to flagellin; TLR6 to zymosan and lipopeptide; and TLR9 to CpG (unmethylated DNA). TLRs are part of the larger superfamily of Toll-IL-1 receptors (TIRs). “Mammals have two kinds of receptors with TIR domains,” Beutler says, “TLRs and receptors for the cytokines IL-1 and IL-18, as well as some orphan receptors.” All TIRs signal by way of TIR domain adapters, such as MyD88 and Trif (see figure). These systems are very ancient. Thus, an ancestral (prevertebrate) TLR may have adopted a pro-inflammatory function as long ago as 500 million years, Beutler and his colleagues speculate. Drosophila and mammalian innate immune sensors achieve specificity in different ways. In Volume 70, Number 7, 2004 / ASM News Y 319 mice, LPS binds to a cell-surface molecule, CD14. Direct exposure of TLR4 to LPS then activates intracellular signaling. “In a CD14 knockout mouse, you can bypass the need for CD14 by increasing LPS concentration,” Beutler says. “Based on this fact, together with genetic complementation studies showing that TLR4 confers species-dependent ligand recognition, we believe that TLR4 has direct contact with LPS.” However, in Drosophila, Toll does not directly engage microbial molecules, but is activated through a cleaved form of the cytokine peptide called spaetzle. Specificity for gram-positive bacteria occurs prior to spaetzle being cleaved, in a peptide that Reichhart and Hoffman named semmelweis. Akira distinguishes two phylogenetically different groups of TLRs. The first includes TLR2 and TLR4, which are expressed on cell surfaces and recognize microbial structures in the blood. The second consists of TLR7, TLR8, and TLR9, which are present in endosomes within the cytoplasm. “Activation of newer receptors takes place after pathogens are phagocytosed and digested in the phago/lysosome,” Akira says. Once Toll-1 or any of the TLRs is activated, intracellular signaling pathways are very close between insects and mammals, according to Reichhart. In mammals, TLR signaling involves activation of one or more of the “TIR adapter proteins.” The adapters relevant to TLR signaling are known as MyD88, Tirap, Trif, and Tram. Most TLRs act through MyD88 alone or through both MyD88 and Tirap, which leads to production of inflammatory cytokines, chiefly TNF-␣. By screening thousands of mice with induced mutations, Beutler’s group recently identified a second, independent pathway for TLR4 that operates through Trif and Tram and leads to synthesis of type I interferons, upregulates costimulatory molecules, and induces nitric oxide synthase (iNOS). TLR3 is activated by dsRNA through Trif alone, whereas mice with mutations in the Trif gene fail to produce type I interferons in response to viral infection. Because dsRNA serves as an adjuvant in Trif mutants, there may be a separate pathway for dsRNA sensing. Links Being Found between Adaptive and Innate Immune Systems The Trif/Tram pathway provides a direct link between TLR4 activation and adaptive immu- 320 Y ASM News / Volume 70, Number 7, 2004 nity. Although purified LPS is a strong adjuvant, its effects are abolished in the mutant mouse strains that Beutler worked with, suggesting that both the inflammatory and the adjuvant effects of LPS flow through TLR4. Finding that mice with mutations in Trif/Tram lack the adjuvant effect of LPS provides a specific post-TLR4 route for this effect. “As one would expect, adjuvants are sensed through TLRs,” Beutler says. Combining the MyD88/Tirap and Trif/Tram pathways provides a biochemical basis for how adjuvants work. Activating the adaptive immune system requires antigen-presenting cells— macrophages and dendritic cells—to express costimulatory molecules such as CD80, CD86, and CD40, and proinflammatory cytokines. When TLR4 recognizes LPS on the surface of macrophages or dendritic cells, it leads to production of cytokines via MyD88/Tirap and costimulatory molecules via Trif/Tram, providing both components for activating T helper lymphocytes of the adaptive immune system. Seeing that the innate and adaptive immune responses are so tightly linked answers a longstanding question, according to Reichhart. “There was always a question of how an adaptive immune system could defend us if it were alone, because adaptive immunity depends on the multiplication of host cells with a generation time of least 12 hours, whereas microbes can divide every 20 minutes,” he says. To cover this lag, the rapidly reactive innate system responds nearly immediately to infectious agents, protecting the host until the slower adaptive system kicks in and eventually also makes memory cells for long-term responses. Some evidence suggests that the initial innate immune process influences the type of adaptive immune response that is generated. “When naive T helper cells are presented with antigens by antigen-presenting cells, they differentiate into two subsets, T helper 1 (Th1) and T helper 2 (Th2),” Akira says. “Th1 cells secrete interferon-␥, which promote mainly cellular immunity, whereas Th2 cells produce IL-4, IL-5, IL-10, and IL-13, and promote mainly humoral immunity.” Akira says that autoimmune diseases such as Crohn’s disease and multiple sclerosis are associated with an abnormally strong Th1 response, whereas allergic diseases seem to involve an abnormally strong Th2 response. Which limb of adaptive imunity predominates may be modulated by the innate immune response. For instance, Akira says, MyD88-deficient mice are skewed toward a Th2 response. Beutler suggests that perhaps this pattern means that the default pathway for adaptive immune development, absent MyD88 signaling, is the Th2 pathway. Reichhart notes that activation of TLR4 by LPS stimulates Th1 activity, while activating TLR2 through schistosomal egg antigen activates Th2 cells. Therapeutic Possibilities for Infectious and Autoimmune Diseases A number of therapeutic possibilities arise from these basic findings. It seems likely that the TLR system links diseases from infection to arthritis to allergy, promising a broad spectrum of therapeutic benefits. For example, Beutler points to two novel approaches for combating infectious diseases. First, he says, blocking TLR signaling with LPS antagonists might prevent sepsis syndrome. Second, people with particular mutations in TLR signaling pathways who develop severe infections might benefit from drugs that would enable them to bypass those defective steps. For instance, one patient with recurrent bacterial infections and whose leukocytes were hyporesponsive to LPS and IL-1 in vitro carries a defective gene for IRAK4 (interleukin-1 receptor-associated kinase). As well, three children from Saudi Arabia with an inherited IRAK4 deficiency are unusually susceptible to pyogenic bacterial infections. And among several patients with systemic meningococcal disease, the TLR4 locus has a highly significant excess of rare coding changes, according to Beutler and his collaborators. Potentially, such patients could be treated with specific agents to help them overcome these deficiencies. Beutler also sees “huge therapeutic possibilities” if endogenous stimuli of TLRs are part of the etiology of autoimmune or inflammatory diseases. “There may be both endogenous and exogenous sources of ligands for TLRs,” Persing of Corixa says. “We are just beginning to understand the full range of those ligands.” He cites work by Akira suggesting that TLR4 may play an important role in inflammatory bowel disease. “The idea is that a change in gut permeability increases access of microbial components such as LPS, which further drives the inflammatory process,” he says. Blocking the proinflam- matory effects of those components at the level of the TLR might reduce inflammation. Additionally, Persing and his colleagues are working with a detoxified lipid A derivative, monophosphoryl lipid A (MLA), which has been tested in more than 40,000 human volunteers as a vaccine adjuvant, and several synthetic lipid A mimetics called aminoalkyl glucosaminide 4-phosphates (AGPs). All appear to operate through TLR4, and changes in structure of the AGPs lead to drastic differences in TLR4 signaling capacity. When mixed with a vaccine protein, these compounds dramatically accelerate and augment the immune response to that protein. “Both MLA and AGPs, when delivered to the airways via aerosol, produce a rather profound level of resistance against infectious challenge with influenza virus and Listeria,” Persing says. “We could essentially protect animals against 5 to 6 LD50’s of both infectious agents. We think the intranasal approach works with LPS-type compounds because TLR4 is one of the few TLRs that appear to be expressed throughout the respiratory tract.” Certain structures of AGPs appear to be a good fit for human TLR4; in mice, these compounds promote protective innate immune responses in the airways against infectious challenge for up to a week. Dosing at weekly intervals maintains protection, but protection for a week at a time theoretically allows the compounds to be used on an “as-needed” basis. Sponsored by a U.S. Army grant, Persing and his colleagues are now preparing one of the AGPs for a dose-escalation study in humans in 2005. “Even before the anthrax scare, the military was interested in broad measures for protection of the airways against infectious challenge,” Persing says. “Military planners believe that the main portal of entry for bioweapons will be the airways and mucosal route.” Such a threat could come from weaponized Bacillus anthracis or other pathogens such as Francisella or Yersinia pestis. Meanwhile, perhaps more imminent threats arise from natural “bioweapons,” including the severe acute respiratory syndrome (SARS) coronavirus and avian strains of influenza. Persing recently was awarded a substantial five-year contract by the National Institute of Allergy and Infectious Diseases to develop agonists of TLRs that can be used to develop rapidacting vaccines. He and his colleagues found Volume 70, Number 7, 2004 / ASM News Y 321 that delivering an AGP along with an influenza virus antigen intranasally produces a strong adjuvant effect along with a mucosal IgA immune response and a systemic IgG response. In the meantime, while the vaccine is taking effect, the AGP component of the vaccine provides shortterm protection against viral infection. By exploiting the protective features of the innate and adaptive responses, the researchers designed a dosing schedule to protect against an influenza viral challenge within a few hours of the first dose. In separate studies, Persing’s group is using these compounds to suppress allergic responses to ragweed. “By manipulating the innate immune response in the context of an allergen challenge,” he says, “we can dramatically reduce the response to the allergen so it is no longer characterized by the allergic phenotype.” Broad Effects, Ancient Lineage The toll-like receptors link a broad spectrum of species. When 17th-century poet and clergyman John Donne wrote, “Therefore never send to ask for whom the bell tolls, it tolls for thee,” he was emphasizing kinship within the human species. Donne could not have imagined a protective system that tolls not only for humans, but for all mammals and invertebrates as well. The Toll/ TLR system offers yet another proof of relatedness across the animal kingdom, as well as providing insights into the workings of evolution. “It is amazing when you understand this homology for the first time,” Reichhart says. “As biologists, however, we are not so surprised that things are going so far back, 600 million years, to the Cambrian explosion. All systems were already present at that time.” Beutler raises an even more extreme possibility—that the Toll/TLR family goes back to plants. “Even plants have TIR domain proteins,” he says. “And wherever you have TIR domains, those domains have defensive functions.” That pushes the origin of these protective proteins back to 1 or 2 billion years ago. Moving from the remote past to the near future, Beutler sees one of the major challenges in the TLR field as discovering how TLRs, the “eyes” of the innate immune system, “see” their microbial quarry. “TLRs engage ligands according to the same rules of protein chemistry that apply to all other receptors,” he says. “In the years to come, we will understand exactly how LPS is bound by TLR4, how DNA is bound by TLR9, and how all of the other TLRs recognize their specific ligands. Then we will understand how such interactions might be mimicked, encouraged, or effectively blocked.” SUGGESTED READING Hoffmann, J. A., and J. M. Reichhart. 2002. Drosophila innate immunity: an evolutionary perspective. Nature Immunol. 3:121–126. Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–983. Persing, D. H., R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldridge, R. M. Hershberg, and S. G. Reed. 2002. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10(Suppl.):32–37. Poltorak, A., X. He, I. Smirnova, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088. Reichhart, J.-M. 2003. TLR5 takes aim at bacterial propeller. Nature Immunol. 4:1159 –1160. Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443– 451. 322 Y ASM News / Volume 70, Number 7, 2004