* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Global phosphorus cycle
Arctic Ocean wikipedia , lookup
Raised beach wikipedia , lookup
The Marine Mammal Center wikipedia , lookup
Indian Ocean wikipedia , lookup
Marine geology of the Cape Peninsula and False Bay wikipedia , lookup
Marine debris wikipedia , lookup
Blue carbon wikipedia , lookup
Physical oceanography wikipedia , lookup
Marine habitats wikipedia , lookup
Marine biology wikipedia , lookup
Abyssal plain wikipedia , lookup
Ecosystem of the North Pacific Subtropical Gyre wikipedia , lookup
Ocean acidification wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Marine pollution wikipedia , lookup
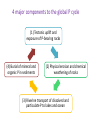
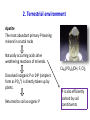
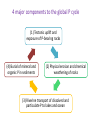
Global phosphorus cycle OCN 623 – Chemical Oceanography 11 April 2013 © 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. 2. 3. 4. Introduction on global phosphorus (P) cycle Terrestrial environment Atmospheric environment Marine environment o o o P in marine sediments P in oceanic water column Oceanic residence time of P 5. P biogeochemistry on long (geologic) time scales 6. Summary 1. Introduction The Global Phosphorus Cycle. Treatise on Geochemistry. 4 major components to the global P cycle (1) Tectonic uplift and exposure of P-bearing rocks (4) Burial of mineral and organic P in sediments (2) Physical erosion and chemical weathering of rocks (3) Riverine transport of dissolved and particulate P to lakes and ocean The Global Phosphorus Cycle. Treatise on Geochemistry. P Reservoirs and Fluxes mol P x 10 12 Atmosphere 0.1 0.0009 0.14 0.01 0.02-0.05 Land Biota Ocean Biota 84-97 1.6 - 4.5 Minable 323-645 2-6.5 2-6.5 19.4-35 19.4-35 0.39-0.45 Land <60cm Rivers .032 diss + 0.6 part 3100-6450 Ocean 0-300m 87.4 0.01 fisheries 0.64 0.3-0.6 Crustal rocks >60cm + marine sediments 0.3 x 108 - 1.3 x 108 Deep Ocean 2,810 1.13-1.4 part 1.87 downwell 2. Terrestrial environment Apatite The most abundant primary P-bearing mineral in crustal rocks Naturally occurring acids drive weathering reactions of minerals. Ca10(PO4)6(OH, F, Cl)2 Dissolved inorganic P or DIP (simplest form as PO43-) is directly taken up by plants. Returned to soil as organic P P is also efficiently sorbed by soil constituents Phosphate is particle reactive! [DIP] in soil waters is maintained low [𝑃]𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 ) [𝑃]𝑠𝑜𝑙𝑖𝑑 KD = ( is low P is efficiently scavenged by: Al(OH)3, Fe(OH)3, and other forms of Al- and Feoxyhydroxides in soils e.g. Fe(OH)3 scavenges P P cycling in rivers • Rivers are the major source of P to the oceans. • Most P in rivers is associated with particulate matter. “PO43- buffer mechanism” Thermodynamic equilibrium between DIP concentration and suspended sediment → maintain constant level of bioavailable P (PO43-) → turbid rivers, e.g. Amazon, Congo, and Orinoco Anthropogenic influence e.g. fertilizer use, deforestation, waste water, etc. → Overall, 50 to 300% increase in riverine P flux to the ocean P cycling in estuaries P removal from water column Flocculation of Fe in low-salinity region Flocculation of humic compounds P addition to water column Remobilization of sorbed P by displacement reactions Anoxic diagenesis in sediments Biological uptake of P Groundwater seepage may be an important source of P to coastal zone. → Not well understood. 3. Atmospheric environment Atmospheric P reservoir and fluxes are small No stable gaseous P compounds Phosphine, PH3, (g): rare Main atmospheric vector P containing dust Important for P-limited regions e.g. Amazon, weathered HI islands, oceanic gyres 4. Marine environment In pelagic sediments P deposition is dominated by secondary P minerals Authigenic carbonate fluorapatite (CFA) a.k.a. francolite In coastal sediments P deposition as detrital P as well The Global Phosphorus Cycle. Treatise on Geochemistry. Coupled Fe-PO4 cycle in marine sediments Fe-redox cycle Provides an effective means of trapping phosphate in sediments Promotes the precipitation of CFA – sink for P Jarvis et al., 1994. Authigenic carbonate fluorapatite (CFA) francolite Dominant P mineral in phosphorite deposits in the ocean Contain ca. 5 – 40 wt. % P2O5 Compare with sedimentary rocks and seafloor sediments = less than 0.3 % wt. % P2O5 Actively mined for production of fertilizer Why ‘carbonate’? Fluorapatite (Ca10(PO4)6F2) incorporates the characteristics of the interstitial pore fluids. “Disseminated” authigenic carbonate fluorapatite CFA diluted with a high concentration of detrital sediment The Global Phosphorus Cycle. Treatise on Geochemistry. Another authigenic phosphate mineral Vivianite, Fe3(PO4)2∙8H2O Formation is restricted to anoxic environments with excess reactive Fe oxyhydroxides. • Leftovers after iron sulfide formation • e.g. deltaic marine environments The Global Phosphorus Cycle. Treatise on Geochemistry. Dissolved inorganic P (DIP) 3 ionic species in seawater: HPO42- (87%) PO43- (12%) H2PO4- (1%) Dissolved organic P (DOP) =Total dissolved P (TDP) - DIP Atlas et al., 1976. Net primary production oceancolor.gsfc.nasa.g ov/FEATURE/gallery.ht ml Estimates of total marine primary productivity Schlesinger, W.H. (1997) Biogeochemistry: An analysis of global change. Academic Press, San Diego. Data from HOT site • Plot (a) Depth 0-100 m (circles); 100-200 m (squares); 200-500 m (triangles) • Plot (b) Depth 0-100 m • Plot (c) Sediment trap-collected particulate matter at 150 m • Redfield ratio = dashed lines Shift in the N:P ratio: >16 Karl et al., 1997. 2 diagnostic parameters for Plimitation (1) Dissolved inorganic N:P ratio (2) Presence of alkaline phosphatase (APase) activity Oceanic P residence time Broecker and Peng (1980) and prior works have estimated Tr (P) = ca. 100,000 years Recent studies have identified new P-sinks (e.g. CFA and other authigenic minerals) Recognition of high burial rates of P in oceanic margins Updated Tr (P) = ca. 10,000 – 17,000 years Short enough for changes in P reservoirs to influence glacial-interglacial CO2 cycles Oceanic P-burial and P residence time fluctuate with sea level Enhanced P burial High sea level, interglacial period shelf slope Abyssal plain Transport to open ocean Low sea level, glacial period shelf slope Abyssal plain 5. P biogeochemistry on geologic time scales 1) Changes in oceanic P inventories can affect atmospheric CO2 levels. Elevated biological productivity → enhanced consumption of surface water CO2 → invasion of atmospheric CO2 P as a limiting nutrient limits CO2 draw-down 2) Assessing paleoceanographic P levels Cd:Ca ratio in benthic forams as a proxy for DIP [Cd] is linearly correlated to [PO4] (DIP) in modern oceans. DOP can be an important, if not the primary, source of P to phytoplankton. May be better to look at the relationship between Cd and TDP Coupled P-Fe-O2 cycles and oxygenation of the atmosphere If oceanic bottom waters are well-oxygenated… Fe2+ oxidizes to form Fe oxyhydroxide precipitates Efficiently scavenge DIP resupplied at the surface water Reduced biological productivity If deep ocean was anoxic and there was little O2 in the atmosphere… (young Earth) Little Fe oxyhydroxide precipitation Larger concentration of oceanic DIP Enhanced biological productivity → maintain atmospheric O2 reservoir 4 major components to the global P cycle (1) Tectonic uplift and exposure of P-bearing rocks (4) Burial of mineral and organic P in sediments (2) Physical erosion and chemical weathering of rocks (3) Riverine transport of dissolved and particulate P to lakes and ocean 6. Summary • Terrigenous (and also aeolian) input of P to ocean • P is efficiently scavenged by Fe oxyhydroxides. • P may be removed from sediments by authigenic mineral formation. • P can be re-mobilized by microbial respiration of organic matter or reductive dissolution of Fe oxyhydroxides. • Shift to P-limitation in oligotrophic open ocean • Changes in P reservoirs can influence glacial-interglacial CO2 cycles and atmospheric O2 levels in geologic time scales.