* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Measuring Temperature
Black-body radiation wikipedia , lookup
Heat exchanger wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Calorimetry wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Conservation of energy wikipedia , lookup
Thermal comfort wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat capacity wikipedia , lookup
Internal energy wikipedia , lookup
Heat equation wikipedia , lookup
Thermal conductivity wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermal radiation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
Heat transfer wikipedia , lookup
Heat transfer physics wikipedia , lookup
Temperature wikipedia , lookup
Thermoregulation wikipedia , lookup
Adiabatic process wikipedia , lookup
Thermal conduction wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
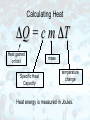
Measuring Temperature The standard metric unit of temperature is the degree Celsius (˚C). Water freezes at 0˚C. Water boils at 100˚C. The Fahrenheit scale is used only in the United States. Why Do We Need Temperature Scales? Our sense of how hot or cold something feels cannot be trusted. Try this! Put one hand in hot water and the other in cold. Then put them both into the same container of warm water. Conflicting messages will be sent to your brain. Absolute Zero When a gas is held at constant pressure and is cooled, its volume decreases by 1/273 for each 1˚C the temperature goes down. By the time you'd get down to a temperature of -273˚C, the volume would decrease to zero. Therefore, -273˚C must be the coldest possible temperature. We call it absolute zero. The Kelvin Scale The zero point on the Kelvin temperature scale is absolute zero. In this way, there are no negative numbers on the Kelvin scale. Scale divisions on the Kelvin scale are identical in size to those on the Celsius scale. Heat and Temperature Heat and temperature are not the same thing. Temperature is related to the average kinetic energy of moving molecules. The faster the molecules move, the higher the temperature. Heat and Temperature Heat depends on temperature, but also on the mass of the object, and its heat capacity. Even though Lake Ontario is at a colder temperature than your cup of coffee, it contains a lot more heat. The reason is that Lake Ontario is so much bigger (more massive) than your morning beverage. Thermodynamics The first law of thermodynamics states that the heat added to a system will show up as an increase in the system's internal energy plus any external work done by the system. A good example of this would be an automobile engine. nd 2 Law of Thermodynamics Heat always flows spontaneously from an area of higher temperature to an an area of lower temperature. (Never the other way around.) In picture (a) the cup holds hot coffee; in picture (b) the cup holds ice water. Thermodynamics The Second Law of Thermodynamics Stated as the Law of Heat Engines: Any cyclic process that uses thermal energy to do work must also have a thermal energy exhaust. In other words, heat engines are always less than 100% efficient at using thermal energy to do work. Thermodynamics All heat engines depend on the spontaneous flow of thermal energy from high temperature to low temperature. There is a limit to how efficient heat engines can be. Their efficiency is theoretically restricted to 60% or less. Thermodynamics Sometimes, the waste heat can be (partially) made use of. An example: A steam-electric power generation plant can make use of its waste thermal energy to heat buildings. It can even be sold to nearby property owners for heating. This is called cogeneration. Waste heat from your car's engine is used to heat the interior of your car on a cold winter day! Things Run Down The 2nd law of Thermodynamics applies to more than just heat engines. When a pendulum swings, each swing is a little lower and slower than the last. Friction keeps shaving off some of its PE and KE and turning it into waste thermal energy which dissipates. The Laws of Thermodynamics & Us When we use the earth's energy resources, we do not decrease the earth's total energy. This is just another way of stating the Law of Conservation of Energy. Instead, we are changing highly useful forms of energy into less useful forms. And this restates the 2nd Law of Thermodynamics. Entropy Suppose a box full of hot gas is touched to a box full of cold gas. cold hot Thermal energy will flow from the high temperature box to the low temperature box. What will it look like after this has happened? Entropy After some time has passed, the temperatures will equalize. The boxes will now look like this: The fast and slow moving molecules are no longer segregated. They are mixed. Did the molecules actually move from one box to the other? Entropy This was the more organized state. This is the more disorganized state. The amount of disorganization is called entropy. Entropy The 2nd Law of Thermodynamics stated as the Law of Entropy: The total entropy (or microscopic disorganization) of the participants in any physical process cannot decrease during that process, but it can increase. That's not to say that you can't create a little more order in some part of your world, but the price you pay is to create more disorder somewhere else. Entropy In other words, disorganization or randomness must increase. Specific Heat Capacity Different substances have different thermal capacities for storing energy. A substance with a low specific heat, (like the pie crust) heats up quickly and cools down quickly because it required little energy to change its temperature. On the other hand, the filling has a high specific heat and is still too hot to eat! Calculating Heat ΔQ = c m ΔT Heat gained or lost Specific Heat Capacity mass temperature change Heat energy is measured in Joules. Thermal Expansion As the temperature of most substances increases, its molecules move faster and farther apart. Most substances expand when heated and contract when cooled. Extreme heat on a July day caused the buckling of these railroad tracks. Thermal Expansion Brass expands more when heated than iron does, and it contracts more when cooled. Because of this behavior, the strip bends. Bi-metallic strips like these are used in thermostats. Water as an Exception Most liquids contract when they turn solid. Water actually expands when it turns to ice. As a result, ice floats on liquid water. background image from photosharingforum.com






























![THERMODYNAMICS [5] Halliday, David, Resnick, Robert, and](http://s1.studyres.com/store/data/002767133_1-7fe915bb6d85222a753bd0cb40b901e8-150x150.png)


