basics of heat transfer
... The descriptions of most scientific problems involve expressions that relate the changes in some key variables to each other. Usually the smaller the increment chosen in the changing variables, the more general and accurate the description. In the limiting case of infinitesimal or differential chang ...
... The descriptions of most scientific problems involve expressions that relate the changes in some key variables to each other. Usually the smaller the increment chosen in the changing variables, the more general and accurate the description. In the limiting case of infinitesimal or differential chang ...
Ch. 1
... The descriptions of most scientific problems involve equations that relate the changes in some key variables to each other. Usually the smaller the increment chosen in the changing variables, the more general and accurate the description. In the limiting case of infinitesimal or differential changes ...
... The descriptions of most scientific problems involve equations that relate the changes in some key variables to each other. Usually the smaller the increment chosen in the changing variables, the more general and accurate the description. In the limiting case of infinitesimal or differential changes ...
Atmospheric stability Dr. Pat Fitzpatrick
... Second main point: if an otherwise stable lifted parcel changes from a gaseous to liquid state (condensation), under certain environmental conditions, the parcel will become unstable. In these specific conditions, latent heat release makes the formerly denser parcel warmer than the environment. It i ...
... Second main point: if an otherwise stable lifted parcel changes from a gaseous to liquid state (condensation), under certain environmental conditions, the parcel will become unstable. In these specific conditions, latent heat release makes the formerly denser parcel warmer than the environment. It i ...
The high temperature heat capacity and related thermodynamic
... Heat capacity data give the solid state physi ...
... Heat capacity data give the solid state physi ...
V. Water Vapour in Air
... More precisely, the air is said to be saturated with respect to a plane surface of pure water at temperature T . The pressure es that is then exerted by the water vapour is called the saturation vapour pressure over a plane surface of pure water at temperature T . Similarly, if the water were repla ...
... More precisely, the air is said to be saturated with respect to a plane surface of pure water at temperature T . The pressure es that is then exerted by the water vapour is called the saturation vapour pressure over a plane surface of pure water at temperature T . Similarly, if the water were repla ...
Equilibrium Chapter 17
... A. No new product is formed by the forward reactions. B. The forward and reverse reactions occur at equal rates. C. The concentration of products is equal to the concentration of reactants. D. All the reactants have been used up. ...
... A. No new product is formed by the forward reactions. B. The forward and reverse reactions occur at equal rates. C. The concentration of products is equal to the concentration of reactants. D. All the reactants have been used up. ...
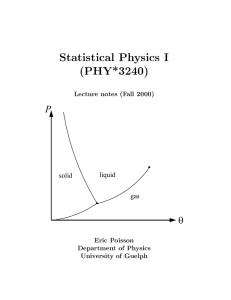
book - University of Guelph Physics
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
Thermodynamics
... can be transformed (changed from one form to another), but cannot be created or destroyed.[25] The first law is usually formulated by saying that the change in the internal energy of a closed thermodynamic system is equal to the difference between the heat supplied to the system and the amount of work ...
... can be transformed (changed from one form to another), but cannot be created or destroyed.[25] The first law is usually formulated by saying that the change in the internal energy of a closed thermodynamic system is equal to the difference between the heat supplied to the system and the amount of work ...
2. Local equilibrium thermodynamics.
... states, it considers neither flows, nor spatial inhomogeneities in simple systems with no externally imposed force fields such as gravity. In the account in terms of equilibrium states of a system, descriptions of irreversible processes refer only to initial and final static equilibrium states; the ...
... states, it considers neither flows, nor spatial inhomogeneities in simple systems with no externally imposed force fields such as gravity. In the account in terms of equilibrium states of a system, descriptions of irreversible processes refer only to initial and final static equilibrium states; the ...
Black-body radiation
Black-body radiation is the type of electromagnetic radiation within or surrounding a body in thermodynamic equilibrium with its environment, or emitted by a black body (an opaque and non-reflective body) held at constant, uniform temperature. The radiation has a specific spectrum and intensity that depends only on the temperature of the body.The thermal radiation spontaneously emitted by many ordinary objects can be approximated as blackbody radiation. A perfectly insulated enclosure that is in thermal equilibrium internally contains black-body radiation and will emit it through a hole made in its wall, provided the hole is small enough to have negligible effect upon the equilibrium.A black-body at room temperature appears black, as most of the energy it radiates is infra-red and cannot be perceived by the human eye. Because the human eye cannot perceive color at very low light intensities, a black body, viewed in the dark at the lowest just faintly visible temperature, subjectively appears grey (but only because the human eye is sensitive only to black and white at very low intensities - in reality, the frequency of the light in the visible range would still be red, although the intensity would be too low to discern as red), even though its objective physical spectrum peaks in the infrared range. When it becomes a little hotter, it appears dull red. As its temperature increases further it eventually becomes blindingly brilliant blue-white.Although planets and stars are neither in thermal equilibrium with their surroundings nor perfect black bodies, black-body radiation is used as a first approximation for the energy they emit.Black holes are near-perfect black bodies, in the sense that they absorb all the radiation that falls on them. It has been proposed that they emit black-body radiation (called Hawking radiation), with a temperature that depends on the mass of the black hole.The term black body was introduced by Gustav Kirchhoff in 1860. When used as a compound adjective, the term is typically written as hyphenated, for example, black-body radiation, but sometimes also as one word, as in blackbody radiation. Black-body radiation is also called complete radiation or temperature radiation or thermal radiation.