* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Down Syndrome: MR Quantification of Brain Structures and
Dual consciousness wikipedia , lookup
Limbic system wikipedia , lookup
Donald O. Hebb wikipedia , lookup
Cortical stimulation mapping wikipedia , lookup
Craniometry wikipedia , lookup
History of anthropometry wikipedia , lookup
Brain damage wikipedia , lookup
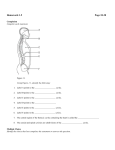
1207 Down Syndrome: MR Quantification of Brain Structures and Comparison with Normal Control Subjects Serge Weis 1 Germain Weber-2·3 Andreas Neuhold 4 Andreas Rett 3 For quantification of brain structures from MR scans, a novel, powerful stereologic tool known as Cavalieri's principle was applied. This tool enables an objective estimation of volume. The method was applied to detect differences in various brain structures between persons with Down syndrome and control subjects. On the basis of absolute values, smaller volumes for the whole brain, cerebral cortex, white matter, and cerebellum were seen in persons with Down syndrome. Similar results were observed when a normalization procedure, based on the volume of cranial cavity, was used. Stereologic determinations of the volumes of brain structures from MR images can reliably identify volume differences between persons with Down syndrome and control subjects. AJNR 12:1207-1211, November/December 1991 Received October 2, 1990; revision requested January 14, 1991 ; final revision received May 13, 1991; accepted May 31 , 1991 . This work was supported by the Ludwig Boltzmann Society, Austria. ' Institute of Neuropathology, University of Munich , Thalkirchnerstrasse 36, D-8000 Munich 2, Germany. Address reprint requests to S. Weis . 2 Institute of Psychology, University of Vienna, Golsdorfgasse 3/2, A-1 010 Vienna , Austria . 3 Ludwig Boltzmann Institute for Research on Brain Damaged Children, Neurological HospitaiRosenhugel , Riedelgasse 5, A-1130 Vienna, Austria. ' Department of Radiology, Hospital Rudolfinerhaus, Billrothstrasse 78, A-1190 Vienna , Austria. 0195-6108/91 /1206-1207 ©American Society of Neuroradiology Morphometry gives reliable , objective, and reproducible data that permit easy comparisons of results derived from brains in different clinical conditions . Stereology, a morphometric discipline, is a body of mathematical methods relating threedimensional parameters defining the structure to two-dimensional measurements obtainable on sections of the structures [1]. By these means, subtle differences in the structure of the brain as found in various clinical conditions can be assessed easily. The ensuing information is clinically valuable for diagnostic decisions and therapeutic interventions. Down syndrome (OS) is known to be one of the major conditions related to mental retardation . DS results from meiotic nondisjunction of chromosome 21 in 95% of cases, from translocation of parts of this chromosome in 3% of cases , and from mosaicism in 2% of cases [2 , 3]. Neuropathologic features of DS are lower brain weight; smaller frontal lobes, brainstem, and cerebellum; and reduced frontoorbital diameter [4] . These descriptions are based on findings derived from brain autopsies; therefore , they are affected by possible postmortem problems like preterminal events, postmortem delay time , fixation , and weighing . All of the problems mentioned can be avoided with the use of axial CT or MR scans. Morphometric data derived from the brains of persons with DS and based on stereologic methods are thus far lacking . The only attempts to quantify brains of living persons with DS have been based on CT measurements [5 , 6]. However, as resolution and delineation of different brain structures on CT scans are limited , morphometric evaluations based on CT scans seem to be inadequate. In contrast , MR scans give higher resolution and allow proper delineation of gray and white matter as well as of brain nuclei [7 , 8] . The present study reports on quantitative data of different structures of brains obtained from MR scans of persons with DS compared with age-matched control subjects. Cavalieri 's principle [9], a novel , powerful stereologic tool for estimating the volumes of brain structures, was applied . Furthermore, problems related to normalization of brain volume to body height were considered, and a normalization 1208 WEIS ET AL procedure based on the volume of the cranial cavity was carried out. AJNR:12 , November/December 1991 For statistical analysis , the one-way analysis of variance (ANOVA) was computed with the software package STATGRAPHICS. Results Subjects and Methods In the investigation group of OS subjects, we studied seven adults , two women and five men 30-45 years old (mean age, 38 years). OS subjects were participants in an interdisciplinary investigation that included medical screening tests as well as psychological assessments (1 0). These seven were recruited from their family homes, and all of them showed a trisomy 21 karyotype originating from chromosomal nondisjunction. The control group comprised seven persons, two women and five men 36-44 years old (mean age, 38 years). Neither neuropathologic nor other clinical symptoms were seen in the control subjects. None were on neuropsychopharmacologic treatment. Imaging was performed on a 0.5-T superconductivity unit (Philips Gyroscan S5) with a spin-echo (SE) technique and an inversionrecovery (IR) technique. With theSE technique, T2-weighted images, 2000/50,100/2 (TRfTEfexcitations), were obtained in axial planes. The IR technique provided T1-weighted images, 1800f400f80/2 (TR/ TlfTEjexcitations), in axial planes. The images obtained with the IR technique had a slice thickness of 8 mm and a slice gap of 1.6 mm. Thus, the slice thickness plus slice gaps equaled 9.6 mm . The imaging matrix was 256 x 256 and the field of view was 250 mm. Volume measurements were based on Cavalieri's principle (9, 11 , 12]: The volume of any object may be estimated from randomized and parallel sections separated by a known distance by summing up the areas of all cross sections of the object and multiplying this sum by the known distance . The MR scans were randomized, since the first slice hitting the brain fell randomly ; these were followed by systematic sections, with the known fixed interval equal to the slice thickness plus slice gap. The first slice was positioned at a distance less than 9.6 mm below the upper margin of the cortical surface. The MR images were fed with a camera into the image analysis system, IBAS 2000 (Kontron , Eching , Germany). The contours of the different brain structures were manually traced with a cursor by one of the authors. Each brain structure was traced three times. A mean value was calculated and subsequently used for statistical analyses. The accuracy of the measurement was verified by calculating the coefficient of error. The profile area of the brain structures was then measured for each slice. The profile areas of each brain structure were summed up and multiplied by slice thickness. The volumes of the following brain structures were determined: cerebral cortex , white matter, ventricles , thalamus. caudate nucleus, lentiform nucleus , cerebellum , and brainstem. The measurement of the ventricles included measurements of the lateral, third, and fourth ventricles. Measurement of the lentiform nucleus included measurement of the putamen and globus pallidus . The volume of the whole brain resulted in summing up the volumes of the previously defined structures . In addition, the volume of the intracranial cavity was determined . The coefficient of error is an indicator for the precision of measurements of individual brain structures . The coefficient of error was calculated for each brain structure. Details regarding the coefficient of error have been published (11 ). The following normalization procedure was used: The volume of the different brain structures was related to the volume of cranial cavity. The volume of the cranial cavity was set at 100% . Values for the volume of the different brain structures were calculated relative to the cranial cavity. Furthermore , we related the volumes of different brain structures to the volume of the whole brain. The volume of the whole brain was set at 100% . Values for the volumes of the different brain structures were calculated relative to the whole brain . On the MR scans of the DS and control groups, no pronounced signs of atrophic changes were seen on the basis of direct visual examination by an experienced neuroradiologist (Fig. 1). Values for the coefficient of error giving the precision of the measurement were not tabulated . They were less than 5% for the following brain structures: cranial cavity, brain, cerebral cortex, white matter, ventricles, thalamus, cerebellum, and brainstem. They ranged between 5% and 10% for the caudate and lentiform nuclei. Thus, accuracy and precision of the measurement were given corresponding to the rules of the coefficient of error and systematic sampling [11 ]. The morphometric data of the different brain structures as measured on T1-weighted MR scans for the investigation and control groups are shown in Table 1. A significantly smaller volume of the whole brain was measured in persons with DS as compared with controls. This difference in volume was attributable predominantly to a reduced volume of cerebral cortex and white matter in DS as compared with controls . The volume of the cerebellum in DS also showed significant differences in comparison with the control group. The other brain structures did not show significant differences between persons with DS and control subjects. Different results were obtained when ratios relating the volume of brain structures either to the volume of the cranial cavity or to the volume of the whole brain were calculated (Table 2). With the use of the proposed normalization procedure based on the volume of the cranial cavity, the ANOVA computations gave approximately the same results as obtained with absolute values (see Table 1). However, when Fig. 1.-Horizontal MR scan of a person with Down syndrome. The following brain structures dispiayed on the scan were analyzed: cerebral cortex, white matter, ventricles, caudate nucleus, lentiform nucleus. AJNR :12, November/December 1991 BRAIN MEASUREMENT ON MR IN DOWN SYNDROME TABLE 1: Absolute Values for Volumes of Different Brain Structures Obtained by Morphometry of MR Scans from Persons with Down Syndrome and Control Subjects Mean Volume in cm 3 (SD) Structure Brain Cerebral corte x White matter Ventricles Thalamus Caudate nucleus Lentiform nucleus Cerebellum Brainstem Cranial cavity Controls (n = 7) Down Syndrome (n = 7) 1313.1 (146.0) 632.5 (65 .1) 462.4 (56.1) 23 .3 (9.1) 16.7 (4 .9) 8.5 (1.7) 14.0 (1 .5) 149.4 (31 .2) 29 .9 (6.2) 1571 .2 (231.0) 1081 .6 (81 .0) 528 .8 (40 .1) 360.3 (51.1) 29 .1 (13.2) 14.3 (6.5) 8.6 (2.5) 14.5 (3 .6) 121.3 (12.5) 24 .4 (3.4) 1443.2 (99.0) F Ratio Value 13.5 12.8 12.6 0.9 0.6 0.0 0.1 4.7 4.0 1.8 .003 .004 .004 .36 .46 .96 .76 .05 .07 .20 p TABLE 2: Normalization Procedures of Brain Structures: Ratios in Down Syndrome and Control Subjects Brain Structure/Whole Brain Brain Structure/Cranial Cavity Structure Controls Brain Cerebral cortex White matter Ventricles Thalamus Caudate nucleus Lentiform nucleus Cerebellum Brainstem 48 .2 35 .2 1.7 1.2 0.6 1.1 11 .3 2.3 Down Down p p Controls Syndrome Value Syndrome Value 48.9 33.3 2.6 1.3 0.8 1.3 11 .2 2.2 .57 .27 .09 .88 .18 .08 .90 .88 83.6 40 .3 29.4 1.5 1.1 0.5 0 .9 9 .5 1.9 75 .0 36 .6 25 .0 2.0 1.0 0.6 1.0 8.4 1.7 .001 .01 .02 .16 .66 .59 .36 .06 .22 relating the volume of brain structures to the volume of the whole brain by relying on these relative values , we were unable to observe any difference between the two groups (Table 2). Discussion Measurements on CT scans have been performed by different investigators [13-19] in an attempt to quantify changes of brain structures occurring with normal and pathologic aging. In most of these investigations, linear, one-dimensional measurements were used to determine specific parameters related to the ventricular system, skull vault, and external CSF spaces. Since the basic rules of stochastic geometry are not followed , meaningful results based on one-dimensional parameters are limited. Priority has to be given to volume estimation as a three-dimensional parameter rather than to linear, one-dimensional parameters [20] . Objective estimations of the total ventricular volume of the brain in 10 hydrocephalic children and adults have been determined recently by stereologic assessment of CT scans [21]. Values of ventricular volume were compared with values obtained by Evan's ratio. Evan 's ratio is derived from the widths of the right and left anterior horns divided by maximum width of the internal skull [13, 22]. The authors of the previous study [21] showed that Evan's ratio is of dubious value . CT and MR scans have been analyzed by semiautomated 1209 image analysis [5, 6, 19, 23-27] . The analysis procedures use the differences in gray level of pixels to differentiate between and delineate brain structures. With some ease, the ventricles and CSF spaces can be resolved on CT. However, the exact boundary of cerebral cortex as well as subcortical nuclei is very difficult to discern from CT scans. Thus , results of morphometric analysis based on CT scans only yield a limited amount of information . MR allows a more exact delineation between gray and white matter, as well as between subcortical nuclei. Recently, semiautomated image analysis procedures have been developed for morphometric analysis of MR brain scans [25-27]. A normalization of brain volume to body height has recently been proposed [28]. These authors rely on data showing that brain size is proportional to body height. Since persons with DS are of smaller stature, the authors performed a normalization of brain volume in the following way: The absolute volume was divided by the subject's height and then multiplied by 170 em, the average height of the male coworkers at their laboratory. However, the relationship between brain size and body height remains obscure, and thus its relevance is questionable. On the basis of an analysis of 3406 autopsy brains, no significant correlation was found between brain weight and body height. The correlation coefficient was given for males as r = .28 and for females as r = .30 [29]. Some authors express their data in relative values or as ratios [5, 15, 17, 27]. Such results must be interpreted very carefully , since it should be kept in mind that, when ratios are used, no significant differences in the structures of interest can be detected when the structure of interest as well as the reference structure show equivalent differences. Both arguments lead to the so-called "reference trap, " which has been discussed in detail [30]. As shown in Table 2, the ratio of the volume of the brain structure to the volume of the brain masked differences that were seen when absolute values were used. It shows that the variable of brain volume is correlated to the volumes of the other brain structures, and hence is not a suitable variable for a normalization procedure in this context. For the normalization procedure, therefore , we introduced a reference variable that is independent. In the sample, it could be pointed out that the volume of the cranial cavity showed no significant differences between the DS and control groups. Thus, when using the volume of cranial cavity as the normalization variable relative to other volumes of brain structures, it was demonstrated that the normalized values showed similar differences between the two groups, as those inferred from the use of absolute volume values . It is interesting that the typical brachycephalic skull form of persons with DS, which was also obvious in our DS subjects, had no influence on the volume of the cranial cavity in comparison with that of the controls (see Table 1). In this study, we could show that the volume of the cranial cavity was an independent variable , as seen in Table 1. However, the volume of the cranial cavity should not be treated as an independent variable per se. Its validity as such a parameter must be determined separately for each study. In another study, the volume of the cranial cavity was measured in a postmortem session by occupying the cranial cavity completely with a lubricated balloon filled with water [31] . It was shown that 1210 WEIS ET AL. the ratio of brain to cranial cavity volume is constant in persons 20-55 years old [31]. The question about the influence of cranial cavity to brain size, and of brain size to cranial cavity, has to be addressed when analyzing changes during developmental stages. In this case each component can have an influence on the other. In the case of hydrocephalus, the overproduction or malabsorption of CSF leads to an accelerated expansion of the brain . Since ossification in this developmental stage is not complete, the bony skull expands in the same way as the brain. The opposite occurs in the case of a premature ossification of the cranial sutures. The bony skull is no longer expanding. The underlying brain is growing but is restricted by a rigid skull. In adults, such cases are not encountered. The sizes of the cranial cavity as well as of the brain have already been determined. The brain is, however, able to regressively change, resulting in brain atrophy. When this occurs, the size of the cranial cavity is not affected. Intracranial space-occupying lesions like tumors, even when they reach a large size, have no expanding influence on the cranial cavity. Considering these points, we are confident that in our study the volume of the cranial cavity was indeed an independent variable . Absolute values of different brain regions derived from CT or MR scans can be compared with those values obtained from autopsy brains. Thus , the reliability of the measurements performed on CT and MR scans can be evaluated. Major discrepancies in volume estimation are found between investigations based on CT techniques [6, 19, 23] and our own investigation, which was performed on MR scans. Comparing the results of other MR investigations [25, 26] and our own investigation, it can be seen that the values derived from MR scans were very close to those values obtained from analysis of autopsy brains [32]. Until now, only a few quantitative CT investigations have been carried out in persons with OS [5, 6, 28, 33]. In these investigations, it was shown that healthy adults with OS had smaller brains and smaller intracranial volumes than did control subjects [5 , 6, 28]. Normalized volumes of CSF, ventricles , and brain parenchyma did not differ between OS and control subjects. Furthermore, both persons with OS and controls showed similar significant age-related incremental increases in volumes of CSF and ventricles [5] . Increased CSF and third ventricular volumes as well as decreased gray and white matter volumes were found in older persons with OS (> 45 years), who showed signs of dementia as compared with younger OS adults [6]. The authors concluded that brain atrophy must be present to accompany dementia in older persons with OS, despite the presence of Alzheimer neuropathology in all older subjects with OS. Furthermore, in persons with OS who are over 50 years old, a significant widening of the temporal horns has been demonstrated recently [33] . The decrease in intracranial size seen in the previous studies [5 , 6, 28] occurred mainly because the authors used only seven CT scans for their measurements. Thus , they were not able to measure the volume of the whole cranial cavity. This may also explain the discrepancies between our results and those reported previously. In the present investigation, data derived by morphometric AJNR:12 , November/December 1991 analysis of MR scans showed differences between persons with OS and control subjects in brain volume, cerebral cortex , white matter, and cerebellum. In addition, measurements of the caudate nucleus, lentiform nucleus, and thalamus are reported. No differences in the volume of these latter structures were found between OS and control subjects. Furthermore, the volume of the brainstem was reduced in subjects with OS in comparison with controls, but this difference did not reach statistical significance. The most striking difference between both groups was smaller volumes of whole brain, cerebral cortex , white matter, and cerebellum. However, persons with OS did not show specific atrophic changes in the brain. It is noteworthy that no differences were found in the volumes of the cranial cavity between persons with OS and control subjects. Despite radiologists' finely developed ability to interpret morphologic patterns visually and intuitively, there are some things they cannot assess by these means, mainly quantitative differences. Volume differences must be on the order of 30-50% to be discernible [34]. The differences in brain volume in persons with OS amounted to 18%, and thus are within a range that can be reliably detected only by morphometry. Cavalieri 's principle for volume estimation of structures is a very powerful stereologic tool and, when applied to welldelineated brain structures obtained from MR scans or autopsy slices, provides objective data. The combination of the techniques of morphometry and MR provides the most suitable method for investigating differences and changes in the CNS in a living population for cross-sectional as well as longitudinal studies. ACKNOWLEDGMENTS We thank H. OrCin and W. Schliesser for technical assistance and S. Ettlinger, Institute of Psychology, University of Vienna, for advice and help with the English-language text. REFERENCES 1. Weibel ER. Stereological methods, vol. 1. London: Academic Press, 1979:1-415 2. Loesch D, Smith CAB. Discriminant diagnosis of 21-trisomy mosaicism. J Ment Defic Res 1976;20 :207-219 3. Cooper DN, Hall C. Down 's syndrome and the molecular biology of chromosome 21. Prog Neurobiol1988;30:507-530 4. Scott BS, Becker LE , Petit TL . Neurobiology of Down 's syndrome. Prog Neurobiol1983 ;21 : 199-237 5. Schapiro MB, Creasey H, Schwartz M, et al. Quantitative CT analysis of brain morphometry in adult Down's syndrome at different ages. Neurology 1987;37 : 1424-1427 6. Schapiro MB, Luxenberg JS, Kaye JA, Haxby JV, Friedland RP , Rapoport Sl. Serial quantitative CT analysis of brain morphometries in adult Down 's syndrome at different ages. Neurology 1989;39 :1349-1353 7. Drayer BP. Imaging of the brain. Part I. Normal findings. Radiology 1988;166: 785-796 8. Wahlund LO , Agartz I, Almquist 0 , et al. The brain in healthy aged individuals: MR imaging. Radiology 1990;174 :675-679 9. Cavalieri B. Geometria degli indivisibili (original edition, Bologna: 1635). Torino: Unione Tipografico-Editrice Torinese, 1966 :1-320 10. WeberG , Rett A. Down Syndrom im Erwa chsenenalter. Bern: Verlag Hans Huber, 1991 : 1-296 AJNR:12 , November/December 1991 BRAIN MEASUREMENT ON MR IN DOWN SYNDROME 11. Gundersen HJG , Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc 1987;147:229-263 12. Cruz-Orive LM . Stereology: recent solutions to old problems and a glimpse into the future. Acta Stereo/1987;6[suppl3]:3-18 13. Gyldensted C. Measurements of the normal ventricular system and hemispheric sulci of 100 adults with computed tomography. Neuroradiology 1977;14: 183-192 14. Meese W, Kluge W, Grumme T , Hopfenmuller W. CT evaluation of the CSF spaces of healthy persons. Neuroradiology 1980;19 :131-136 15. Yamaura H, Ito M, Kubota K, Matsuzawa T. Brain atrophy during aging: a quantitative study with computed tomography. J. Gerontal 1980;35 : 492-498 16. Takeda S, Matsuzawa T. Age-related brain atrophy: a study with computed tomography. J Geronto/1985;40 : 159-163 17. Yerby MS , Sundsten JW, Larson EB, Wu SA, Sumi SM. A new method of measuring brain atrophy? The effect of aging in its application for diagnosing dementia. Neurology 1985;35 :1316-1320 18. Massman PJ , Bigler ED, Cullum CM , Naugle Rl. The relationship between cortical atrophy and ventricular volume./nt J Neurosci 1986;30 :87-99 19. Schwartz M, Creasey H, Grady CL, et al. Computed tomographic analysis of brain morphometries in 30 healthy men, aged 21 to 81 years. Ann Neuro/1985;17:146-157 20. Weis S, Haug H, Holoubek B, brun H. The cerebral dominances: quantitative morphology of the human cerebral cortex. lnt J Neurosci 1989;47 :165-168 21 . Pakkenberg B, Boesen J, Albeck M, Gjerris F. Unbiased and efficient estimation of total ventricular volume of the brain obtained from CT-scans by stereological method . Neuroradiology 1989;31 :413-417 22 . Evans WA. An encephalometric ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatry 1942;47:931-937 23. Creasey H, Schwartz M, Frederickson H, Haxby JV, Rapoport Sl. Quantitative computed tomography in dementia of the Alzheimer type. Neurology 1986;36 : 1563-1568 24. Deleo JM , Schwartz M, Creasey H, Cutler N, Rapoport Sl. Computed- 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 1211 assisted categorization of brain computerized tomography pixels into cerebrospinal fluid , white matter, and gray matter. Comput Biomed Res 1985;18:79-88 Filipek PA, Kennedy DN, Caviness VS , Rossnick SL, Spraggins TA, Starewicz PM . Magnetic resonance imaging-based brain morphometry: development and application to normal subjects. Ann Neurol 1989;25 : 61-67 Caviness VS , Filipek PA, Kennedy DN . Magnetic resonance technology in human brain science: blueprint for a program based upon morphometry. Brain Dev 1989;11: 1-13 Jernigan TL, Press GA, Hesselink JR. Methods for measuring brain morphologic features on magnetic resonance images: validation and normal aging. Arch Neuro/1990;47:27-32 Schapiro MB , Haxby JV, Grady CL, Rapoport Sl. Cerebral glucose utilization, quantitative tomography , and cognitive function in adult Down syndrome. In: Epstein CJ , ed . The neurobiology of Down syndrome. New York: Raven, 1986:89-108 Rbthig W, Schaarschmidt W. Lineare Zusammenhange zwischen Kbrperlange und Hirnmasse. Gegenbaurs Morpho/ Jahrb 1977;123 :208- 213 Braendgaard H, Gundersen HJG. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods 1986;18 :39-78 Davis PJM , Wright EA. A new method for measuring cranial cavity volume and its application to the assessment of cerebral atrophy at autopsy. Neuropathol Applied Neurobiol 1977 ;3 : 341-358 Eggers R, Haug H, Fischer D. Preliminary report on macroscopic age changes in the human prosencephalon. A stereologic investigation . J Hirnforsc h 1984;25: 129- 139 LeMay M, Alvarez N. The relationship between enlargement of the temporal horns of the lateral ventricles and the dementia in aging patients with Down syndrome. Neuroradiology 1990;32 :104-107 Reith A, Mayhew TM . Stereo/ogy and morphometry in electron microscopy. New York: Hemisphere, 1988 :1-2 15