* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Sudden cardiac death and variant angina
Survey
Document related concepts
Heart failure wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac surgery wikipedia , lookup
Drug-eluting stent wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiac arrest wikipedia , lookup
Transcript
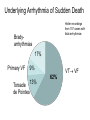
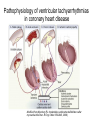
REVIEW Sudden cardiac death and variant angina R4 최은용 / Pf. 김원 Sudden Cardiac Death is one of the Leading Causes of Death in the U.S. 300,000 250,000 200,000 150,000 100,000 50,000 0 AIDS Breast Cancer Lung Cancer Stroke Source: Statistical Abstract of the U.S. 1998, Hoover’s Business Press, 118th Edition SCA SCD definition • Sudden cardiac death (SCD) natural death due to cardiac causes time and mode of death are unexpected 1 h or less, unwitnessed deaths(death within 24h) -2/3 of SCDs heart disease - Incidence : 400,000 - 500,000/year in U.S. Only 2% - 15% reach the hospital Half of these die before discharge High recurrence rate may be reversible cardiovascular collapse, cardiac arrest, and death Incidence of SCD Incidence Events per Year Adult population CAD History of a coronary event Heart failure MADITII Resuscitation SCD-HeFT AVID,CIDS,CASH Resuscitation with previous MI MADJTI,MUSTT 0 1 2 5 10 PERCENT 20 50 0 100000 200000 300000 ABSOLUTE NUMBER Sudden cardiac death in Hispanic Americans and African Americans. Am J Public Health 87:1461, 1997.) Myerburg et al., Circulation 1992 Age related risk of SCD Modified from Myerburg and Castellanos 2008 Time-dependent risk of SCD after MI Sudden cardiac death: Structure, function, and time-dependence of risk. Circulation 85[Suppl I]:I2, 1992 Etiology Sudden cardiac death • Causes – 80-90% are tachyarrhythmias – Only 10-20% are due to an acute myocardial infarction or to bradyarrhythmias Underlying Arrhythmia of Sudden Death Holter recordings from 157 cases with fatal arrhythmias Bradyarrhythmias 17% Primary VF 9% Torsade 13% de Pointes Bayes de Luna et al. Am Heart J 1989 VT VF 62% Coronary artery Abonormalities Atherosclerotic Coronary Artery Disease • Cronary artery abnormality : 80% of SCDs in Western countries nonischemic cardiomyopathies 10% to 15% • Atherosclerotic Coronary Artery Disease electrophysiologic alterations that result from the myocardial impact of an ischemic burden -modulation by hemodynamic, autonomic, genetic -short-term risk of life-threatening events: acute ischemia - longer term risk : transient ischemia, myocardial scarring, remodeling, ischemic cardiomyopathy, and heart failure. Pathophysiology of ventricular tachyarrhythmias in coronary heart disease Modified from Myerburg RJ: Implantable cardioverter-defibrillators after myocardial infarction. N Engl J Med 359:2245, 2008.) Pathology • CHD as the major structural etiologic factor • More than 80% of SCD • combination atherosclerosis of CA and unstable coronary artery lesions • 70–75% male SCD healed MIs, Only 20–30% recent acute Mis • transient ischemia mechanism of onset, electrophysic alterance regional or global left ventricular (LV) hypertrophy often coexists with prior MIs Nonatherosclerotic Coronary Artery Abnormalities • • • • • • • Congenital lesions, coronary artery embolism Anomalous Origin of Coronary Arteries Coronary Arteritis Kawasaki disease Polyarteritis nodosa Mechanical Obstruction to Coronary Arteries Deep myocardial bridges • Coronary Artery Spasm serious arrhythmias and SCD concomitant CAD : Painless myocardial ischemia Prediction and Prevention of Cardiac Arrest and Sudden Cardiac Death • High risk subgroups provide more focused • Effective prevention of underlying diseases • Classified as primary and secondary: multiple ICD trial primary prevention secondary prevention Risk of sudden death by decile of multivariate risk . J Am Coll Cardiol 5[Suppl 6]:141B, 1985. • profiling for risk of developing CHD and risk factor control • long-term risk factors : age, cigarette smoking, elevated serum cholesterol, diabetes mellitus, elevated blood pressure, LV hypertrophy, and nonspecific electrocardiographic abnormalities, elevated CRP not sufficiently or specifically enough to warrant therapies targeted to potentially fatal arrhythmias Primary prevention • After coronary artery disease additional strategies for risk profiling • initial 6–18 months after the event and then plateaus nonsudden, diluting the potential benefit of strategies targeted specifically to SCD. Erly after MI ICD ? Primary prevention • Subgroups at high absolute risk of SCD post MI acute phase: 48 h, risk ~ 15%, not at risk for recurrent cardiac arrest convalescent phase : 3 days to ~6 weeks, episode of sustained VT or VF(large infarct), motality >25% at 12 months, ½ SCD Aggressive intervention chronic phase: : predicted by a number of factors -extent of myocardial damage -EF and/or HF EF <40%. -VT or VF during EPS , EF <35 or 40% strong predictor of SCD risk. implantable cardioverter defibrillators (ICDs) Secondary prevention • Surviving victims of a cardiac arrest Not acute MI or a transient risk of SCD (e.g., drug exposures, correctable electrolyte imbalances) Multivessel CAD and DCMP (↓ EF )1- to 2-year risk of recurrence SCD or cardiac a rrest 30% life-threatening arrhythmias with long QT syndromes or right ventricular dysplasia Clinical Characteristics of Cardiac Arrest Prodrome, Onset, Arrest, Death • Prodrome: angina, dyspnea, palpitations, easy fatigability, not specific for predicting SCD • HR ↑and for advanced grades of PVCs to evolvenonsustained or sustained VT VF • Prognosis: pulseless VT>VF >asystole, PEA primary and secondary cardiac arrests( ddx by hemodynamic instability):90%vs 70% Treatment: Cardiac Arrest (1) initial evaluation and basic life support (2) public access defibrillation (when available) (3) advanced life support (4) postresuscitation care (5) long-term management ADVANCED CARDIAC LIFE SUPPORT (ACLS) (1) defibrillation/cardioversion and/or pacing, (2) intubation with an endotracheal tube, and (3) insertion of an intravenous line Defibrillation Immediate defibrillation should precede intubation and insertion of an intravenous line VF or VT 300 J monophasic or 120–150 J with a biphasic maximum of 360 J monophasic (200 J biphasic) Epi: 1 mg failed defibrillation: 3–5 min Vasopressin : a single 40-unit dose given IV Acidosis (not correted with defibrilation and intubation ) NaHCO3: 1 meq/kg initially and an additional 50% of the dose repeated every 10–15 min; not be used routinely antiarrhythmic therapy - amiodarone (150 mg over 10 min, followed by 1 mg/min for up to 6 h and 0.5 mg/min thereafter) - lidocaine; VF in the early phase of an acute coronary syndrome, amiodarone unsuccessful: a bolus of 1 mg/kg , repeated in 2 min POSTRESUSCITATION CARE • Hypothermia to reduce metabolic demands and cerebral edema • outcome noncardiac diseases is poor, survival rate of <10% sepsis, cancer, CRF, pnuemonia vs transient airway obstruction, electrolyte disturbances, proarrhythmic effects of drugs, and severe metabolic abnormalities LONG-TERM MANAGEMENT AFTER SURVIVAL OF OUT-OF-HOSPITAL CARDIAC ARREST • without irreversible damage to the CNS and hemodynamic stability: diagnostic testing therapeutic interventions (cardiac arrest is followed by a 10–25% mortality rate during the first 2 years after the event) • significant survival benefits ICD: motality 20–35% reduction over 2–5 yr not associated with an ACS MI with an ejection fraction less than 30–35% HCMP, DCMP, rare inherited disorders (e.g., right ventricular dysplasia, long QT syndrome, Brugada syndrome, catecholaminergic polymorphic VT, and so-called idiopathic VF Prevention of SCD in High-Risk Individuals Without Prior Cardiac Arrest • Post-MI patients 30 days or more after the MI EFs <35% - Markers of risk such as ambient ventricular arrhythmias, inducible ventricular tachyarrhythmias in EPS Hx of heart failure very low EFs (e.g., <20%) may receive less benefit Prinzmetal’ s variant angina − Spontaneous angina with ST elevation − First described in 1930s (not by Prinzmetal first) − Transient, abrupt reduction in luminal diameter without preceding increased demand − Normal or diseased vessel, usually within 1cm of plaque − Reversed by nitroglycerin or calcium channel blocker Clinical characteristics − Younger, Associated with migraine, Raynaud’s, cocaine, cigarettes, thyroid disease − Can be precipitated by exercise, hyperventilation − Circadian: midnight to early morning − Arrhythmias: VT, block − MI: usually with underlying diseased vessel − Silent ischemia − Small elevations of troponin Pathogenesis − Autonomic nervous system: Precipitated by acetylcholine / methacholine, Prevented by atropine and alpha blockers − Endothelial dysfunction: increased endothelin release and activity, relation to low estradiol levels May lead to vessel damage, stasis, thrombosis − hypercontractility of vascular smooth muscle due to vasoconstrictor mitogens, leukotrienes, or serotonin. Diagnosis − ECG: ST elevation with chest discomfort -> baseline with resolution 61% have normal ECGs on ED arrival − Exercise stress myocardiac cintigraphy : limited sensitivity − Dobutamine echo: may provoke vasospasm, limited sensitivity − Hyperventilation with ECG changes (6 minutes): 62% sensitive Angiography (Class Iia indication): − usually RCA − multivessel: migratory, sequential at 2 different sites, or simultaneous Ergonovine: − 50 -> 400 micrograms until max dose or positive result (normal arteries will respond diffusely with >400) − − 4 /1000 refractory spasm or VF (only used in those with nl vessels) stress echo: compares well with angiography as gold standard (93% sens / 91% spec) Treatment − Nitrates and calcium channel : treat acute episodes and to abolish recurrent episodes of PVA − Avoid nonselective beta blockers − Aspirin : result of the exquisite sensitivity of coronary tone to modest changes in the synthesis of prostacyclin − Coronary revascularization : helpful discrete, proximal fixed obstructive lesions Prognosis − Many acute, active phase, with frequent episodes during the first 6 months − Long-term survival at 5 years: 90–95% − Nonfatal MI : ~20% by 5 years − Develop serious arrhythmias during spontaneous episodes of pain higher risk for SCD − Condition stabilizes, and there is a tendency for symptoms and cardiac events to diminish over time Variant angina and VT Am J Cardilol 1996:88355-360 Circulation 1979,60:1343`13 Indian Heart J. 2009; 61:389-391 ECG • variant angina: ST elevation ddx : ST segment depression( subendocardial may occur with spasm of smaller epicardial vessels • ECG changes during spontaneous episodes of variant angina by Chierchia and colleagues (1980) peaking of the T waves ST segment elevation, with T wave inversion Over the following days the inverted T waves will progressively become less deep normal morphology T wave is inverted, the T wave will rapidly become upright and recommence the sequence with peaking of the T wave. • "pseudonormalization." (1) transient Q waves; (2) increasing R wave voltage; and (3) ST segment alterans • Ambulatory ECG studies: ddx exertional angina: (1) an absence of a precipitating tachycardia prior to the ischemic changes; and (2) a circadian pattern of disease activity with most ischemic episodes occurring in the early morning, even though they may be asymptomatic (silent ischemia) • exercise stress testing would usually be negative30 to 60 percent of patients. (1) coexistent atherosclerotic coronary artery disease (2) an increased sensitivity to catecholamines during the hot phase of the disorder.