* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Tracer Development for Molecular Imaging
Signal transduction wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Drug discovery wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biosynthesis wikipedia , lookup
Drug design wikipedia , lookup
Citric acid cycle wikipedia , lookup
Technetium-99m wikipedia , lookup
SNP genotyping wikipedia , lookup
Butyric acid wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Biochemistry wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
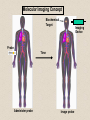
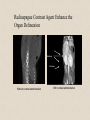
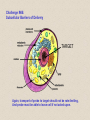
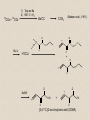
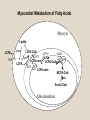
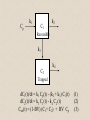
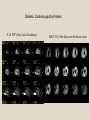
Molecular Imaging Concept Biochemical Target Imaging Device Probe Time Administer probe Image probe Diagnostic Utility Sensitivity Ability to either detect the probe signal at the target (direct response) or change in a signal that is dependent on the quantity of probe at the target (indirect response) Specificity Ability to distinguish the target from nontarget processes or tissues Molecular Imaging Modalities • Gamma ray emission •Positron emission – annihilation photons (PET) •Single photon emission (SPECT) •Direct signal from tissue in response to probe concentration • Gamma ray absorption •Used in CT scans • Contrast with high atomic number nuclei that absorb gamma rays (Iodine) • Magnetic resonance • Protons provide signal in clinical MRI scans • Perturb proton relaxation with Gd contrast agents • Use paramagnetic nuclei (e.g., 13C, 19F) labeled probes •Optical •Fluorescent molecules (luciferase/luciferin; GFP) •Visible wavelengths have limited depth of detectability •Infrared extends depth a bit •Ultrasound •Acoustic absorption/modification (microbubbles) •Thermoacoustic stimulation (absorption probes) CT Contrast Agents • High atomic number – x-ray absorption – Ba, I, Gd, Au • Intraintestinal or intravascular (extracellular) Examples: Barium sulfate (oral, rectal admin.) iopromide (iodinated IV contrast agent) Radioapague Contrast Agent Enhance the Organ Delineation Stomach liver Kidneys Ovarian Without contrast administration With contrast administration Opportunities in microCT:Anatomy and physiology Catheter 700 600 vena cava Enhancement in HU 500 400 kidney 300 input (heart) muscle 200 100 kidne y spine 0 0 50 100 150 200 250 -100 heart vena cava Time in seconds The representative CT images of a dynamic sequence with iodinated contrast enhancement. From top to the bottom, one can see the flowing path of the contrast medium within the blood stream: from tail vein to vena cava, to heart, and then to kidney. These three images are associated with the first 8 seconds of the sequence. Proton MR Contrast Agents • Positive contrast agents (appearing bright on MRI) Small molecular weight compounds containing as their active element Gadolinium (Gd), Manganese or Iron Unpaired electron spins in their outer shells and long relaxivities, which make them good T1 relaxation agents. Examples: GD-DTPA, Gadopentetate dimeglumine, gadoteridol, and gadoterate meglumine are utilized for the central nervous system and whole body Mangafodipir trisodium for lesions of the liver Gadodiamide for the central nervous system. Proton MR Contrast Agents • Negative contrast agents (appearing predominantly dark on MRI) are small particulate aggregates often termed superparamagnetic iron oxide (SPIO). These agents produce predominantly spin-spin relaxation effects, but very small particles smaller than 300 nm also produce substantial T1 relaxation. • A special group of negative contrast agents (appearing dark on MRI) are perfluorocarbons because their presence excludes the hydrogen atoms responsible for the signal in MR imaging. MR Molecular Probes • Some MR contrast agents require biocompatible carriers/capsules – Reduce toxicity – hide the bad guy inside – Target specific cells/proteins/processes Examples: Ferumoxide – SPIO core particles (~150nm), dextran T10 covering Nanomag – SPIO particles (50nm) with cross-linked dextran and amino acid sequences to form bonds to organic compounds P7228 – SPIO, anionic dextran layer – can be encapsulated by positively charged liposomes Biologically Important NMR Nuclei 1H - Wall thickness, ejection fraction, wall motion, perfusion, coronary artery angiography. (large signal from ~50M concentration in tissues) 31P – ATP, PCr, P , PDE, PME, pH , [Mg2+], kinetics of i i creatine kinase and ATP hydrolysis. 23Na – Transmembrane Na+ gradient, tissue and cartilage structure. 13C – Glycogen, metabolic rates, substrate preference, drug metabolism, etc. 19F – Drug metabolism, pH, Ca2+ and other metal ion concentration, pO2, temperature, etc. 2H – Perfusion, drug metabolism, tissue and cartilage structure. In vivo detection sensitivity limits use of C-13 and F-19 molecular probes (C-13 requires >0.1mM, F-19 >5 mM) Advantages of PET • PET has high sensitivity (~pmol of probe can be detected) • PET images biochemistry. Small radionuclides (C-11, F-18) label small biological molecules with retention of biological specificity. • PET images are quantitative General Aspects of PET Tracers • Understanding of targeted biochemical process • Practical synthesis: sufficient yield and purity, automated • Tissue uptake and kinetics are specific to targeted process • Fate of radiolabel understood for metabolized tracers • Tracer distribution is sensitive to answer clinical questions relevant to diagnosis, prognosis or monitoring of therapy • Tissue kinetics amenable to mathematical modeling to give quantitative indices Positron Decay A A Z X N Z1Y N1 nuclide C-11 N-13 O-15 F-18 I-124 + e half-life 20.3 min 10 min 124 sec 110 min 4.2 d (+ high Energy photon) e.g., 18F 18O + e+ + Biochemical/Physicological Targets for PET Imaging • • • • • • • • • • Substrate metabolism carbohydrates, fatty acids, amino acids, oxygen, nucleosides, oligonucleotides Receptor binding adrenergics, cholinergics, neurotransmitters, hormone receptors, growth factor receptors Ionic transport Na, K, Ca, F, Cl, I Perfusion water, ammonia, butanol pH Blood volume (11CO, C15O) Hypoxia (misonidazoles) Redox potentials Protein-protein interactions monoclonal antibodies Gene expression reporter genes and probes Challenge #1: Radiochemical limitations •Short radionuclide half-life (<2 hr) •Limited radionuclide availability •Radiation exposure to chemist >90% of PET probes are synthesize by simple 1 or 2 step labeling followed by purification and formulation Synthesis times typically under 45 min for C-11 (t 1/2 = 20 min) and under 2 hr for F-18 (t 1/2 = 110 min) Challenge #2: Biochemical Complexity TARGET Challenge #3: The radioimaging signal is chemically nonspecific *Probe *(Intermediates) *TARGET *Nonspecific binding to proteins or membranes *alternative binding or metabolic products *systemic metabolites (i.e. hepatic) Specificity! Specificity! Specificity! Challenge #4: Physiological Barriers To Delivery of Probe to the Target Arterial Blood TARGET (Subcellular compartmentation may also limit delivery) Transport of probe to target should not be rate-limiting Limits utility of technique in poorly perfused tissues Challenge #4B: Subcellular Barriers of Delivery TARGET Again, transport of probe to target should not be rate-limiting, And probe must be able to leave cell if not acted upon. 11C-Acetylene • as PET Probe and Labeling Intermediate 11C-acetylene (C2H2) may be useful as a radiolabeling intermediate for organic molecules in physiology studies • [11C]C2H2, by itself, can be used in perfusion studies (i.e. brain) Comparison of PET Tracers for Measuring Tissue Perfusion Tracer Physical half-life Octanol/Water partition coeff. (log P) [15O]water [13N]ammonia [11C]CH3F [18F]CH3F [11C]C2H2 [11C]butanol [15O]butanol 2.01 9.97 20.4 109.8 20.4 20.4 2.01 -1.38 -1.38 0.51 0.51 0.37 0.88 0.88 Solubility in water (g/ 100 ml) 34 0.23 0.23 0.106 7.8 7.8 Cost per synthesis* Ease of Synthesis High Low Moderate Moderate Low Moderate Moderate Simple Simple Difficult Moderate Simple Moderate Difficult * Using proton accelerator and most common nuclear reaction for production 11 CO2 + 12CO2 1) Trap on Ba 2) 900 C / H2 Ba*CC (Madsen et al., 1981) *CCH 2 O O * N O Bu-Li O O O O H*CCLi + O O * O O NaOH OH * O O * + OH O [3,4-11C]-2-oxo-butynoic acid (COBA) Computer-controlled Apparatus for synthesis of C-11 Acetylene Thermocouple Wires Trap Outlet Trap inlet Sieve Trap Dose Calibrator C 0 C-11 CO2 V1a 0 V1b 1 0 0 V2 1 C Recirculation Pump 1 1 Waste C Waste Quartz Rxn Vessel V4 a b d Furnace c V3 Soda Lime Trap Helium Hydrogen Sampling Bag 0 V5 C 1 Draw Sample for Analysis Dose Calibrator Number of Ions Collected (Millions) Gas Chromatography/ Mass Spec of C-11 Acetylene Product Standard Product 4 3 2 1 0 0 1 2 3 4 5 6 Time (min) 7 8 9 Radioactivity (Arbitrary Units) Gas Chromatography / Rad. Detection of C-11 Acetylene Product 5 4 3 2 1 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Time (min) Target: Myocardial Fatty Acid Oxidation • Long-chain fatty acids are the predominant substrates for production of ATP in heart. • Abnormalities of fatty acid oxidation by the myocardium are associated with ischemic heart disease, congestive heart failure, cardiomyopathies, and deficiencies of carriers, enzymes or co-factors required for fatty acid transport or oxidation. • The lack of a specific radiolabeled probe of fatty acid oxidation has impeded the development of a non-invasive technique for assessment of fatty acid oxidation. Myocardial Metabolism of Fatty Acids Myocyte Lipids LCFA FATr lipase LCFA-CoA hyd. LCFA CPT-I LCFA-carn ACS VLAD CPT-II LCFA-CoA b-ox. MTP CAT LCFA-carn MCFA-CoA b-ox. Acetyl-CoA Mitochondrion 6-Thia Analogs 4-Thia Analog 18 F S OH O 14-[18F]fluoro-6-thia-heptadecanoic acid (14F6THA) O 18 F S OH 17-[18F]fluoro-6-thia-heptadecanoic acid (17F6THA) O 18 F S OH 16-[18F]fluoro-4-thia-hexadecanoic acid (FTP) O 18 F O 18 F Myocyte O S (LC-AcylCoA synthetase) (LC-AcylCoA hydrolase) O 18 F S Outer Membrane S CoA Complex Lipids (acyl transferase) (CPT-I) O 18 F S O Mitochondrion Carn (Translocase, CPT-II) Inner Membrane O 18 F S (VLC-acylCoA dehydrogenase) O 18 F Plasma O S S S S CoA (Mit. Trifunctional Protein) CoA slow18 F OH O S S CoA (spontaneous) Protein Binding 18 F SH 1 8 E f f e c t s o f C P T I I n h i b i t i o n o n F F T P B i o d i s t r i b u t i o n i n F a s t e d R a t s a t 3 0 m i n p . i . 1 . 5 C o n t r o l E t o m o x i r t r e a t e d * p < 0 . 0 5 v e r s u s c o n t r o l 1 . 0 Uptake(%dosg/) 0 . 5 * * * * * Heart Blod Lung Brain Liver Kidney BS one Musclekelta 0 . 0 O R G A N Kinetics of [18F]FTP in Isolated Rat Heart 4 0 3 0 N o r m o x i c 2 0 ADV(ml/gdry) H y p o x i c 1 0 0 0 2 4 6 8 1 0 1 2 1 4 1 6 1 8 2 0 T i m e ( m i n ) Cp k1 C1 k2 Reversible k3 k4 C2 Trapped dC1(t)/dt = k1 Cp(t) - (k2 + k3) C1(t) dC2(t)/dt = k3 C1(t) - k4 C2 (t) Ctot(t) = (1-BV) (C1+ C2) + BV Cp (1) (2) (3) 2-Compartment Model Fit to FTP Kinetics in Isolated Rat Heart B V = 0 . 9 8 m l / g d r y 4 0 k = 5 . 0 1 ( m l / m i n / g d r y ) 1 k = 0 . 5 6 / m i n 2 k = 0 . 3 6 / m i n 3 3 0 k = 0 . 0 0 5 0 / m i n 4 D a t a M o d e l ADV(ml/gdry) 2 0 1 0 0 0 2 4 6 8 1 0 1 2 1 4 1 6 1 8 2 0 T i m e ( m i n ) 1 8 S e n s i t i v i t y o f [ F ] L a b e l e d T h i a F a t t y A c i d O x i d a t i o n R a t e t o H y p o x i a I n I s o l a t e d P e r f u s e d R a t H e a r t ( F l o w = 7 m l / m i n ) N o r m o x i c ( 9 5 % O ) 2 H y p o x i c ( 3 5 % O ) 2 3 2 . 5 *p<0.01 2 . 0 * 2 * 1 . 5 0 . 5 FractionlOxdationRe(ml/ingdry) 1 1 . 0 * 0 . 0 0 P a l m i t a t e P a l m i t a t e 1 7 F 6 T H A F T P F O O 18 S OH 18 F S OH F-18 FTP in Normal Human Subject Short-axis Images of Heart at 50-55 min p.i. Duke University Medical Center F 1 8 F T P K i n e t i c s i n N o r m a l H u m a n S u b j e c t 6 5 4 3 2 L i v e r R e n a l C o r t e x H e a r t ( s e p t u m ) B l o o d P o o l F T P I n p u t F u n c t i o n B r a i n l u n g %dose/10ml 1 . 0 8 6 5 4 3 2 0 . 1 8 6 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 T i m e ( m i n ) F 1 8 F T P N o n m e t a b o l i z e d F r a c t i o n Nonmetabolized fraction of F-18 FTP in Plasma N o r m a l H u m a n S u b j e c t ( 1 1 1 8 1 9 9 8 ) 1 0 . 9 0 . 8 0 . 7 y = 1 ( A x / ( B + x + C / x ) ) 0 . 6 A = 0 . 6 5 3 , B = 1 . 7 3 7 , C = 1 1 2 . 1 Fraction 0 . 5 0 . 4 0 . 3 0 . 2 0 . 1 0 051 0 1 5 2 0 2 5 3 0 T i m e ( m i n ) 2 C o m p a r t m e n t M o d e l F i t t o F T P K i n e t i c s i n N o r m a l H u m a n M y o c a r d i u m 2 0 0 0 . 0 1 8 0 0 . 0 D a t a M o d e l 1 6 0 0 . 0 1 4 0 0 . 0 1 2 0 0 . 0 T B V = 0 . 2 6 ; k 1 = 0 . 1 6 4 ; k 2 = 0 . 0 6 9 ; k 3 = 0 . 0 7 0 4 ; k 4 = 0 . 0 0 5 F R = 0 . 0 8 2 7 ( m l b l o o d / m i n / m l t i s s u e ) F T P nci/ 1 0 0 0 . 0 8 0 0 . 0 6 0 0 . 0 4 0 0 . 0 2 0 0 . 0 0 . 0 0 1 0 2 0 3 0 4 0 T i m e ( m i n ) Diabetic Cardiomyopathy Patient F-18 FTP (Fatty Acid Oxidation) SPECT Tc-99m Myoview Perfusion Scan F 1 8 F T P K i n e t i c s 2 C o m p a r t m e n t M o d e l F i t D i a b e t i c , I s c h e m i c C a r d i o m y o p a t h y ( 1 1 8 1 9 9 9 ) 0 . 7 D a t a M o d e l 0 . 6 0 . 5 nci/ 0 . 4 0 . 3 T B V = 0 . 3 2 3 ; k 1 = 0 . 1 2 8 ; k 2 = 0 . 8 1 1 ; k 3 = 0 . 2 0 6 ; k 4 = 0 . 0 0 0 ( f i t ) 0 . 2 0 . 1 F R = 0 . 0 2 8 0 ( m l b l o o d / m i n / m l t i s s u e ) F T P 0 . 0 0 1 0 2 0 3 0 4 0 T i m e ( m i n )