* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Stahl_3rd_ch19_Part2..
Aging brain wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Biology and sexual orientation wikipedia , lookup
Biology of depression wikipedia , lookup
Neuroeconomics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Hypothalamus wikipedia , lookup
Synaptic gating wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Sexual dysfunction wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neurotransmitter wikipedia , lookup
Actions of Acamprosate in the VTA:
Reducing Excessive Glutamate Release to Relieve Withdrawal
VTA
9- glu
6
/J!J. JA opiate receptor
VSCC
'W" GABA
o
<J
~
mGluR receptor
m
NMDA receptor
en kephalin
Jt
GABA-B receptor
DA
t1
GABA-A receptor
FIGURE 19-29
Actions of acamprosate in the ventral tegmental area (VTA). Acamprosate seems to block
glutamate receptors, particularly metabotrophic glutamate receptors (mGluRs) and perhaps also N-methyl-daspartate (NMDA) receptors. When alcohol is taken chronically and then withdrawn, the adaptive changes that it
causes in both the glutamate system and the GABA system create a state of glutamate overexcitation as well as
GABA deficiency. By blocking glutamate receptors, acamprosate may thus mitigate glutamate hyperexcitability
during alcohol withdrawal.
978
I
Essential Psychopharmacology
Actions of Opiates on Reward Circuits
nucleus
accumbens
VTA
FIGURE 19-30 Actions of opiates on reward circuits. Neurons originating in the arcuate nucleus project to both
the ventral tegmental area (VTA),site of dopamine cell bodies and also where many neurotransmitters project,
and the nucleus accumbens, to which dopaminergic neurons project. Opiate neurons release endogenous opiates
such as enkephalin.
which may last several hours, followed in turn by drowsiness ("nodding"),
mood swings,
mental clouding, apathy, and slowed motor movements. In overdose, these same agents act
as depressants
reversed
of respiration
by synthetic
and can also induce coma. The acute actions of opiates can be
opiate antagonists
such as naloxone
and naltrexone,
as antagonists at opiate receptors.
When given chronically, opiates easily cause both tolerance
which
and dependence.
compete
Adapta-
tion of opiate receptors occurs quite readily after chronic opiate administration.
The first
sign of this is the patient's need to take higher and higher doses of opiate in order to relieve
pain or induce the desired euphoria. Eventually,
that causes euphoria and that which produces
sign that dependence
their sensitivity
has occurred
to agonist
actions
there may be little room between
the toxic effects of an overdose.
and that opiate receptors
is the production
have adapted
of a withdrawal
the dose
Another
by decreasing
syndrome
Disorders of Reward, Drug Abuse, and Their Treatment
once the
I 979
Endogenous Opiate Neurotransmitters
FIGURE 19-31
Endogenous opiate neurotransmitters.
Opiate drugs act on a variety of receptors called opiate
receptors, the most important of which are mu, delta, and kappa. Endogenous opiate-like substances are peptides
derived from precursor proteins called POMC (pro-opiomelanocortinJ, proenkephalin, and prodynorphin. Parts of
these precursor proteins are cleaved off to form endorphins, enkephalins, or dynorphin, which are then stored in
opiate neurons and presumably released during neurotransmission to mediate reinforcement and pleasure.
chronically administered opiate wears off. Opiate antagonists such as naloxone can precipitate a withdrawal syndrome in opiate-dependent persons. This syndrome is characterized by
feelings of dysphoria, craving for another dose of opiate, irritability, and signs of autonomic
hyperactivity such as tachycardia, tremor, and sweating. Piloerection ("goose bumps") is
often associated with opiate withdrawal, especially when a drug is stopped suddenly ("cold
turkey"). This is so subjectively horrible that the opiate abuser will often stop at nothing in
order to get another dose of opiate to relieve such symptoms. Thus, what may have begun
as a quest for euphoria may end up as a quest to avoid withdrawal. Clonidine, an alpha 2
adrenergic agonist, can reduce signs of autonomic hyperactivity during withdrawal and aid
in the detoxification process.
In the early days of opiate use/abuse/intoxication and prior to the completion of the
neuroadaptive mechanisms that mediate opiate receptor desensitization, opiate intoxication
in the abuser alternates with normal functioning. Later, after the opiate receptors adapt and
the person becomes dependent, he or she may experience very little euphoria but mostly a
state of lack of withdrawal alternating with the presence of withdrawal.
Treatment of opiate dependence
Opiate receptors can readapt to normal if given a chance to do so in the absence of additional intake of drug. This may be too difficult to tolerate, so reinstituting another opiate,
980
Essential Psychopharmacology
Methadone
opiate full agonist
FIGURE 19-32
Buprenorphine
opiate partial agonist (OPA)
Icons of methadone and buprenorphine. Methadone, a full agonist at opiate receptors, and
buprenorphine, a partial agonist at opiate receptors, are both used during detoxification from exogenous opiates
such as codeine, morphine, and heroin.
such as methadone, which can be taken orally and then slowly tapered, may assist in the
detoxification process (Figure 19-32). A partial mu opiate agonist, buprenorphine, now
available in a sublingual dosage formulation combined with naloxone, can also substitute
for stronger full agonist opiates and then be tapered. It is combined with the opiate naloxone, which does not get absorbed orally or sublingually but prevents intravenous abuse,
since injection of the combination of buprenorphine plus naloxone results in no high and
may even precipitate withdrawal. L-alpha-acetylmethodol acetate (LAAM) is a long-acting
orally active opiate with pharmacological properties similar to those of methadone, but it
is rarely used because of concerns over QTc prolongation. Agonist substitution treatments
are best used in the setting of a structured maintenance treatment program that includes
random urine drug screening and intensive psychological, medical, and vocational services.
For heavy-duty opiate addicts, specialty methadone clinics may be useful.
As for other areas of drug abuse, rank-and-file office-based psychopharmacologists use
very little of the agonist substitution process for treating opiate abusers or addicts, including
relatively little use of buprenorphine. This situation is likely the product of therapeutic
nihilism for opiate addicts and probably also the wish not to have heroin and serious
intravenous opiate addicts in one's practice, since they often must live on the streets, tend to
engage in criminal activities, and are highly unreliable. However, for motivated prescription
opiate addicts who are still employed, reliable, and less likely to be involved with crime or
living on the street and who have not taken methadone previously, buprenorphine may
remain a viable option.
Stimulants
Use of stimulants as therapeutic agents is discussed extensively in several other chapters.
Mechanism of action of amphetamine as an inhibitor of the dopamine transporter (DAT)
and also of the vesicular monoamine transporter (VMAT) is introduced in Chapter 4 and
illustrated in Figure 4-15. Therapeutic use of stimulants for sleep/wake disorders is discussed
in Chapter 16 and illustrated in Figures 16-3, 16-5, 16-31, and 16-32. Therapeutic use
of stimulants for ADHD is discussed in Chapter 17 and illustrated in Figures 17-8, 17-9,
17-18,17-19, and 17-20.
Disorders of Reward, Drug Abuse, and Their Treatment
I
981
Actions of Stimulants on Reward Circuits
nucleus
accumbens
stimulants
VTA
FIGURE 19-33 Actions of stimulants on reward circuits. Shown here is the reactive reward system consisting of
the ventral tegmental area (VTA), site of dopamine cell bodies which also receives many neurotransmitter
projections; the nucleus accumbens, to which dopaminergic neurons project; and the amygdala (far left), which
has connections with both the VTA and the nucleus accumbens. The potential abuse properties of stimulants
stem from their ability to enhance dopamine release in the nucleus accumbens.
Stimulants
and reward
The mechanisms of enhanced reinforcement and abusability of stimulants when given in
high pulsatile doses are compared and contrasted with the reduced reinforcement of stimulants when given in oral sustained release dosing in Figures 17-18 through 17-20. Although
many therapeutic actions of stimulants are thought to be directed to the prefrontal cortex
and also to the enhancement of both norepinephrine and dopamine neurotransmission
there, the actions of stimulants that are linked to their abuse are thought to be primarily
those that target reward circuits, especially dopamine release from mesolimbic dopamine
neurons in the nucleus accumbens (Figure 19-33).
One stimulant without recognized therapeutic uses in psychopharmacology is cocaine
(Figure 19-34). This agent has two major properties: it is both a local anesthetic and an
982
I
Essential PSYChopharmacology
FIGURE 19-34
Icon of cocaine. The
Cocaine
main mechanism of action of cocaine is
DA
to block reuptake and cause the release
of monoamines, principally dopamine
(DA) but also norepinephrine (NE) and
serotonin (5HT). There is also a local
anesthetic action (caine).
NE
5HT
caine
inhibitor of monoamine transporters, especially for dopamine (i.e., DAT, the DA transporter). But often neglected in discussions of cocaine is consideration of its ability to inhibit
the serotonin transporter (SERT) and the norepinephrine transporter (NET) (Figure
19-34). Cocaine's local anesthetic properties are still used in medicine, especially by earnose-and-throat specialists (otolaryngologists). Freud himself exploited this property of
cocaine to help dull the pain of his tongue cancer. He may have also exploited the second
property of the drug, which is to produce euphoria, reduce fatigue, and create a sense of
mental acuity due to inhibition of dopamine reuptake at the dopamine transporter.
Cocaine inhibits DAT in a manner similar to the action of methylphenidate. That is,
cocaine is a blocker of transport of DA by DAT. Cocaine does not act upon dopamine
neurons as amphetamine does, but methamphetamine
(Figure 19-35) acts just like
amphetamine, only faster. In addition, methamphetamine is converted to amphetamine.
Amphetamine and methamphetamine are both pseudosubstrates and reverse transporters of
DA via DAT and also inhibitors ofVMAT. These properties of amphetamine are discussed
in Chapter 4 and illustrated in Figure 4-15.
So what is the difference between methylphenidate and cocaine if they both have
the same mechanism of action? Similarly, what is the difference between amphetamine for
ADHD and methamphetamine for abuse? The answers are not in differences in mechanism
of action but in route of administration and therefore how fast, how powerfully, and how
completely DAT is blocked.
Methylphenidate is taken orally and is longer in onset and duration of action compared
to stimulants that are injected, snorted intranasally, or smoked. Methylphenidate itself is
Disorders of Reward, Drug Abuse, and Their Treatment
I
983
Amphetamine
Methamphetamine
FIGURE 19-35
Icon of amphetamine/
methamphetamine. Amphetamine and
methamphetamine are both
pseudosubstrates and reverse
transporters of dopamine via the
dopamine transporter (OAT) and also
act as inhibitors of the vesicular
monoamine transporter (VMAT).
far more abusable when injected; but even injected, methylphenidate does not seem to blast
the DAT as ferociously as do other stimulants injected intravenously. Cocaine is not active
orally, so users have learned over the years to take it intranasally, where the drug rapidly
enters the brain directly, bypassing the heart, and thus can have a more rapid onset than
even with intravenous administration. The most rapid and robust way to deliver drugs to
the brain is to smoke those that are compatible with this route of administration, as this
avoids first-pass metabolism through the liver and is somewhat akin to giving the drug by
intra-arterial/in tracarotid bolus.
As stated in previous chapters, blasting DAT in a dramatic pulsatile manner maximizes
the chances for phasic DA release (see Figure 17-20), which is highly reinforcing and
pleasurable when this happens in the nucleus accumbens. Taking huge oral doses of a
stimulant with rapid onset, or taking it intranasally, intravenously, or smoking it can create
highly intense pleasurable experiences that are often described by addicts as better than
orgasm.
At intoxicating doses of cocaine or methamphetamine, however, undesirable effects can
be produced, including tremor, emotional lability, restlessness, irritability, paranoia, panic,
and repetitive stereotyped behavior. At even higher doses, these stimulants can induce
intense anxiety, paranoia, and hallucinations, with hypertension, tachycardia, ventricular
irritability, hyperthermia, and respiratory depression. In overdose, cocaine can cause acute
heart failure, stroke, and seizures.
Long-term effects of stimulant abuse
Even worse for DA neurons than high doses may be effects of repetitive intoxicating doses
of stimulants on these neurons. The progression of stimulant abuse is shown in Figure
19-36. First doses of stimulants cause pleasurable phasic dopamine neuronal firing (Figure 19-36A). Eventually, reward conditioning occurs, causing craving between stimulant
doses and lack of pleasurable phasic dopamine firing, with only residual tonic dopamine
neuronal firing (Figure 19-36B). Now that the user is addicted, higher and higher doses
984
Essential Psychopharmacology
Progression of Stimulant Abuse
I® enduring I
cognitive loss
"burn~out"
time
B, C, D, E, and F Progression of stimulant abuse. (A) First doses of stimulants such as
amphetamine or cocaine cause pleasurable phasic dopamine firing. (B) With chronic use, reward conditioning
causes craving between stimulant doses and only residual tonic dopamine firing with lack of pleasurable phasic
dopamine firing. (C) In this addicted state, higher and higher doses of stimulants are needed in order to achieve
the pleasurable highs of phasic dopamine firing. (D) Unfortunately, the higher the high, the lower the low, and
between stimulant doses the individual experiences not only the absence of a high but also withdrawal symptoms
such as sleepiness and anhedonia. (E) The effort to combat withdrawal can lead to compulsive use and
impulsive, dangerous behaviors in order to secure the stimulant. (F) Finally,there may be enduring if not
irreversible changes in dopamine neurons, including long-lasting depletions of dopamine levels and axonal
degeneration, a state that clinically and pathologically is appropriately called "burn-out."
FIGURE 19-36A,
must be taken to get better
and better
"highs" that accompany
phasic dopamine
neuronal
firing (brainwashed;
Figure 19-36C). Unfortunately,
the higher the high, the lower the
low, and between stimulant doses the experience is that of sleepiness and anhedonia,
not
just the absence of a high (withdrawal;
Figure 19-36D). To avoid this state and get ever
more satisfYing highs, the situation progresses to compulsive use, often with marathon,
indiscriminate,
and unprotected
sex, risk of HIV from shared needles and sex, with the
emergence
of paranoia
(Figure
19-26E).
Indeed,
stimulants
can produce
a paranoid
psy-
chosis indistinguishable
from acute paranoid schizophrenia,
as discussed in Chapter 9 and
illustrated in Figures 9-25 and 9-26. At this stage, the addict is often involved with criminal
activity in order to obtain drugs and with people and situations that trigger violence (Figure
19-36E).
Finally, there may be enduring
if not irreversible
changes
in dopamine
neurons,
including long-lasting
depletion of dopamine levels and axonal degeneration,
a state that
clinically and pathologically
is appropriately
called "burn-out"
(Figure 19-36F). This can
be associated with enduring cognitive loss and treatment-resistant
depression, and it can
take a very long time, sometimes years, to reverse if at all.
Although there are no approved treatments for stimulant
there
may be a cocaine
vaccine
(TA-CD)
in the future
abusers or stimulant
that removes
addicts,
the drug before
it
can lead the patient along the route of progression
of stimulant abuse shown in Figure
19-36. The weak DAT inhibitor modafinil is also being tested, as are various antipsychotics
Disorders of Reward, Drug Abuse, and Their Treatment
I 985
such as olanzapine, but particularly D2 partial agonists such as aripiprazole. Experimental
treatments include dopamine D3 receptor partial agonists (RGH188, BP987) or antagonists
(NGB2904, SB277011A, ST198) and long-lasting dopamine releasers such as PAL287.
Naltrexone is also being investigated. N-acetyl cysteine, a precursor of the amino acid
cysteine, acts on the cysteine-glutamate exchange mechanism - which may be disordered
in cocaine-dependent individuals seeking drugs - to reduce craving and interest in cocaine.
Sedative hypnotics
Sedative hypnotics include barbiturates and related agents such as ethchlorvynol and ethinamate, chloral hydrate and derivatives, and piperidinedione derivatives such as glutethimide
and methyprylon. Experts often include alcohol, benzodiazepines, and Z drug hypnotics
in this class as well. The mechanism of action of sedative hypnotics is basically the same
as that of those described in Chapter 14 and illustrated in Figures 14-20 and 14-22 for
the action ofbenzodiazepines: namely, they are positive allosteric modulators (PAMs) for
GABA-A receptors. Actions of sedative hypnotics at GABA-A receptor sites in reward
circuits are shown in Figure 19-37. Molecular actions of all sedative hypnotics are similar,
but benzodiazepines and barbiturates seem to work at different sites from each other and
also only on some GABA-A receptor subtypes, namely those with alpha 1, alpha 2, alpha
3, or alpha 5 subunits (Figure 19-38A). Barbiturates are much less safe in overdose than
benzodiazepines, cause dependence more frequently, are abused more frequently, and produce much more dangerous withdrawal reactions. Apparently the receptor site at GABA -A
receptors mediating the pharmacological actions of barbiturates (Figure 19-38A) is even
more readily desensitized with even more dangerous consequences than the benzodiazepine
receptor (also shown in Figure 19-38). The barbiturate site must also mediate a more intense
euphoria and a more desirable sense of tranquility than the benzodiazepine receptor site.
Since benzodiazepines are generally an adequate alternative to barbiturates, psychopharmacologists can help to minimize abuse of barbiturates by prescribing them rarely if ever. In
the case of withdrawal reactions, reinstituting and then tapering the offending barbiturate
under close clinical supervision can assist the detoxification process.
Marijuana
You can indeed get stoned without inhaling (Figure 19-39)! Actions of marijuana and
its active ingredient THC (delta-9-tetrahydrocannabinol)
on reward circuits are shown in
Figure 19-39 at sites where endogenous cannabinoids are utilized naturally as retrograde
neurotransmitters. The concept of the "brain's own marijuana" is introduced in Chapter 3
and retrograde neurotransmission with these endogenous cannabinoids (or "endocannabinoids") at CB1 presynaptic cannabinoid receptors is illustrated in Figure 3-3.
Cannabis preparations are smoked in order to deliver cannabinoids that interact with
the brain's own cannabinoid receptors to trigger dopamine release from the mesolimbic
reward system (Figure 19-39). Marijuana can have both stimulant and sedative properties. In usual intoxicating doses, it produces a sense of well-being, relaxation, a sense of
friendliness, a loss of temporal awareness including confusing the past with the present,
slowing of thought processes, impairment of short-term memory, and a feeling of achieving special insights. At high doses, marijuana can induce panic, toxic delirium, and rarely
psychosis. One complication oflong-term use is the "amotivational syndrome" in frequent
users. This syndrome is seen predominantly in heavy daily users and is characterized by
the emergence of decreased drive and ambition, thus "amotivation." It is also associated
986
I
Essential Psychopharmacology
Actions of Sedative Hypnotics and Benzodiazepines
on Reward Circuits
nucleus
accumbens
VTA
sedative
hypnotics!
benzodiazepines
FIGURE 19-37
Actions of sedative hypnotics and benzodiazepines on reward circuits. Shown here is the
reactive reward system consisting of the ventral tegmental area (VTA) , site of dopamine cell bodies that receives
many neurotransmitter projections; the nucleus accumbens, to which dopaminergic neurons project; and the
amygdala (far left), which has connections with both the VTA and the nucleus accumbens. Sedative hypnotics
and benzodiazepines are positive allosteric modulators at GABA-A receptors (such as in the VTA, as shown here).
with other socially and occupationally impairing symptoms, including a shortened attention span, poor judgment, easy distractibility, impaired communication skills, introversion,
and diminished effectiveness in interpersonal situations. Personal habits may deteriorate,
and there may be a loss of insight and even feelings of depersonalization. In terms of chronic
administration to humans, tolerance to cannabinoids has been well documented, but the
question of cannabinoid dependence has always been controversial. The discovery of the
brain cannabinoid CEI receptor antagonist rimonabant has settled this question in experimental animals because it precipitates a withdrawal syndrome in mice chronically exposed
to THC. It is therefore highly likely but not yet proven that dependence also occurs in
humans, presumably due to the same types of adaptive changes in cannabinoid receptors
Disorders of Reward, Drug Abuse, and Their Treatment
987
Binding Sites for Sedative Hypnotic Drugs
chloride
channel
GABA
binding site
BZ
binding site
~
barbiturate
binding site
A
benzodiazepine
receptors:
D(1, D(2, D(3, D(
5 subtypes
chloride
channel
neurosteroid binding site
? alcohol binding site
general anesthetics
benzodiazepine
receptors: 6subtypes
(alpha 4, alpha 6)
B
FIGURE
19-38A and B Binding sites for sedative hypnotic drugs. (A) Benzodiazepines and barbiturates both act
at GABA-A receptors, but at different binding sites. Benzodiazepines do not act at all GABA-A receptors; rather,
they are selective for the alpha 1,2,3,
and 5 subtypes. (B) General anesthetics, alcohol, and neurosteroids may
bind to other types of GABA-A receptors.
988 I
Essential Psychopharmacology
Actions of Marijuana and THC on Reward Circuits
nucleus
accumbens
VTA
THC
FIGURE 19-39 Actions of marijuana and THC on reward circuits. Shown here is the reactive reward system
consisting of the ventral tegmental area (VTA), site of dopamine cell bodies that receives many neurotransmitter
projections; the nucleus accumbens, to which dopaminergic neurons project; and the amygdala (far left), which
has connections with both the VTAand the nucleus accumbens. Marijuana delivers its active ingredients, the
cannabinoids (e.g., THC; delta-9-tetrahydrocannabinol), which interact with the brain's own cannabinoid
receptors to trigger dopamine release in the nucleus accumbens.
that occur in other neurotransmitter
of abuse.
There
receptors
are two known cannabinoid
after chronic
receptors,
CEI
administration
of other drugs
(in brain) and CE2 (predominantly
in the immune system). CEI receptors may mediate not only marijuana's reinforcing properties but also those of alcohol and to some extent those of other psychoactive substances
(including food, if sugars and fats can be considered "psychoactive"; this is discussed further
on). Anandamide
is one of the endocannabinoids
and a member of a chemical class of neurotransmitter
that is not a monoamine,
not an amino acid, and not a peptide: it is a lipid,
specifically a member of a family of fatty acid ethanolamides.
Anandamide
shares most but
not all of the pharmacological
properties
of THC,
since its actions
at brain cannabinoid
Disorders of Reward, Drug Abuse, and Their Treatment
I
989
receptors are not only mimicked by TH C but also antagonized in part by the selective brain
cannabinoid CBl receptor antagonist rimonabant.
The discovery of rimonabant, a "marijuana antagonist," has opened the door to using
this drug as a potential therapeutic agent in various types of drug abuse, from cigarette
smoking to alcoholism to marijuana abuse. Additionally, rimonabant has been extensively
tested in obesity and the metabolic syndrome and has received approved for this use in some
countries. However, approval in the United States has not been granted yet due to concerns
about the possibility that long-term use of rimonabant might increase suicidal ideation.
Hallucinogens
The hallucinogens are a group of agents that act at serotonin synapses in the reward
system (Figure 19-40). They produce intoxication, sometimes called a "trip," associated
with changes in sensory experiences, including visual illusions and hallucinations and an
enhanced awareness of both external and internal stimuli and thoughts. These hallucinations are produced with a clear level of consciousness and a lack of confusion and may be
both psychedelic and psychotomimetic. "Psychedelic" is the term for a heightened sensory
awareness and subjective experience that one's mind is being expanded, that one is in union
with all humanity or the universe, and that one is having a sort of religious experience. "Psychotomimetic" means that the experience mimics a state of psychosis, but the resemblance
between a trip and psychosis is superficial at best. As previously discussed, the stimulants
cocaine and amphetamine much more genuinely mimic psychosis.
Hallucinogen intoxication includes visual illusions, visual "trails" where the image
smears into streaks of its image as it moves across a visual trail, macropsia and micropsia, emotional and mood lability, subjective slowing of time, the sense that colors are heard
and sounds are seen, intensification of sound perception, depersonalization and derealization, yet retaining a state of full wakefulness and alertness. Other changes may include
impaired judgment, fear oflosing one's mind, anxiety, nausea, tachycardia, increased blood
pressure, and increased body temperature. Not surprisingly, hallucinogen intoxication can
cause what is perceived as a panic attack, which is often called a "bad trip." As intoxication
escalates, one can experience an acute confusional state (delirium) of disorientation and
agitation. This can evolve further into frank psychosis, with delusions and paranoia.
Common hallucinogens include two major classes of agents. The first class resemble serotonin (indo1ea1ky1amines) and include the classic hallucinogens LSD (d-1ysergic
acid diethylamide), psilocybin, and dimethyltryptamine
(DMT) (Figure 19-41). The
second class of agents resemble norepinephrine and dopamine and are also related
to amphetamine (pheny1a1ky1amines); they include mescaline DOM (2,5-dimethoxy-4methy1amphetamine) and others. More recently, synthetic chemists have come up with
some new "designer drugs" such as MDMA (3,4-methy1ene-dioxymethamphetamine)
and
"Foxy" (5-methoxy-diisopropyltryptamine).
These are either stimulants or hallucinogens
and produce a complex subjective state sometimes referred to as "ecstasy," which is also
what abusers call MDMA itself. MDMA produces euphoria, disorientation, confusion,
enhanced sociability, and a sense of increased empathy and personal insight.
Hallucinogens have rather complex interactions at neurotransmitter systems, but one of
the most prominent is a common action as agonists at 5HT2A receptor sites (Figure 19-42).
Hallucinogens certainly have additional effects at other 5HT receptors (especially 5HT1A
somatodendritic autoreceptors and 5HT2C receptors) and also at other neurotransmitter
systems, especially norepinephrine and dopamine, but the relative importance of these other
990
I
Essential Psychopharmacology
Actions of Hallucinogens on Reward Circuits
nucleus
accumbens
hallucinogens
\
VTA
FIGURE 19-40
Actions of hallucinogens on reward circuits. Shown here is the reactive reward system
consisting of the ventral tegmental area (VTA), site of dopamine cell bodies that receives many neurotransmitter
projections; the nucleus accumbens, to which dopaminergic neurons project; and the amygdala (far left), which
has connections with both the VTA and the nucleus accumbens. Hallucinogens act at serotonin synapses within
this reward system.
actions is less well known. MDMA also appears to be a powerful inhibitor of the serotonin
transporter (SERT) (Figure 19-42) and is also a releaser of 5HT. MDMA and several other
drugs structurally related to it may even destroy serotonin axon terminals. However, the
action that appears to explain a common mechanism for most of the hallucinogens is the
stimulation of 5HT2A receptors.
Hallucinogens can produce incredible tolerance, sometimes after a single dose. Desensitization of 5HT2A receptors is hypothesized to underlie this rapid clinical and pharmacological tolerance. Another unique dimension of hallucinogen abuse is the production of
"flashbacks," namely the spontaneous recurrence of some of the symptoms of intoxication
that lasts from a few seconds to several hours, but in the absence of recent administration
of the hallucinogen. This occurs days to months after the last drug experience and can
Disorders of Reward, Drug Abuse, and Their Treatment
I 991
FIGURE 19-41
Serotonergic Hallucinogens
MDMA
Icons of hallucinogens.
Hallucinogens such as lysergic acid
diethylamide (LSD), mescaline,
psilocybin, and 3,4-methylenediosymethamphetamine (MDMA) are agonists
at serotonin 2A (5HT2A) receptors.
V
5HT2A
psilocybin
LSD
5HT2A
5HT2A
mescaline
o
5HT2A
FIGURE 19-42
5HT neuron
Actions of
hallucinogens. The primary action of
hallucinogenic drugs such as LSD,
mescaline, psilocybin, and MDMA are
shown here: namely, agonism of 5HT2A
receptors. Hallucinogens may have
additional actions at other serotonin
receptors (particularly 5HTlA and
5HT2C) and at other neurotransmitter
systems, and MDMA in particular also
blocks the serotonin transporter (SERT).
992 I
Essential Psychopharmacology
apparently be precipitated by a number of environmental stimuli. The psychopharmacological mechanism underlying flashbacks is unknown, but its phenomenology suggests the
possibility of a neurochemical adaptation of the serotonin system and its receptors related
to reverse tolerance that is incredibly long-lasting. Alternatively, flashbacks could be a form
of emotional conditioning embedded in the amygdala and then triggered when a later emotional experience, occurring when one is not taking a hallucinogen, nevertheless revives the
memory of experiences that occurred during intoxication. This could precipitate a whole
cascade of feelings that occurred during intoxication with a hallucinogen. This is analogous to the types of reexperiencing flashbacks that occur without drugs in patients with
posttraumatic stress disorder.
Club drugs
Phencyclidine
and ketamine
Phencyclidine (PCP) and ketamine both have actions at glutamate synapses within the
reward system (Figure 19-43). They both act as antagonists of NMDA receptors, binding to a site in the calcium channel (see discussion in Chapter 13 and Figures 13-30 and
13-31). Both were originally developed as anesthetics. PCP proved to be unacceptable for
this use because it induces a unique psychotomimetic/hallucinatory
experience very similar
to schizophrenia. The NMDA receptor hypoactivity that is caused by PCP has become
a model for the same neurotransmitter abnormalities postulated to underlie schizophrenia (see discussion in Chapter 9 and Figures 9-39 through 9-42). Its structurally and
mechanism-related analog ketamine is still used as an anesthetic, but it causes far less of the
psychotomimetic/hallucinatory
experience. Nevertheless, some people do abuse ketamine,
one of the "club drugs" sometimes called "special K." PCP causes intense analgesia, amnesia,
and delirium, stimulant as well as depressant actions, staggering gait, slurred speech, and
a unique form of nystagmus (i.e., vertical nystagmus). Higher degrees of intoxication can
cause catatonia (excitement alternating with stupor and catalepsy), hallucinations, delusions,
paranoia, disorientation, and lack of judgment. Overdose can include coma, extremely high
temperature, seizures, and muscle breakdown (rhabdomyolysis).
Gamma hydroxybutyrate
(GHB)
This agent is discussed extensively in Chapter 16 as a treatment for narcolepsy/cataplexy. It
is sometimes also abused (Figure 19-43). The mechanism of action ofGHB is as an agonist
at its own GHB receptors and at GABA-B receptors (illustrated in Figure 16-35).
Inhalants
Agents such as toluene are thought to be direct releasers of dopamine in the nucleus accumbens.
Sexual disorders
From a psychopharmacological perspective, the human sexual response can be divided into
three phases, each with distinct and relatively nonoverlapping neurotransmitter functions:
libido, arousal, and orgasm (Figure 19-44).
Libido
The first stage, libido, is linked to desire for sex, or sex drive, and is a complex process regulated by neurotransmitters, hormones, and past experiences. Dopamine activity in reward
circuitry is thought to playa central role (Figure 19-45). In addition to the projections of
Disorders of Reward, Drug Abuse, and Their Treatment
I
993
Actions of Club Drugs on Reward Circuits
nucleus
accumbens
PCP
ketamine
GHB
FIGURE 19-43 Actions of club drugs on reward circuits. Shown here is the reactive reward system consisting of
the ventral tegmental area (VTA),site of dopamine cell bodies that receives many neurotransmitter projections;
the nucleus accumbens, to which dopaminergic neurons project; and the amygdala (far left), which has
connections with both the VTAand the nucleus accumbens. Club drugs such as phencyclidine (PCP) and
ketamine are antagonists at N-methyl-d-aspartate (NMDA) receptors and thus cause NMDAhypoactivity, which
in turn leads to disinhibition of dopamine release. Gamma hydroxybutyrate (GHB), which is an agonist at GHB
and GABA-Breceptors, is also sometimes abused.
dopamine to the nucleus accumbens emphasized
in this chapter, dopamine also projects
to the hypothalamus,
where it may also have input to the regulation of sexual desire via
neurons
in the medial preoptic
to play an important
area
(MPOA
in Figure
role in sexual motivation
19-45). The MPOA
in experimental
animals,
has been shown
whereas
another
area of the hypothalamus,
the paraventricular
nucleus, may control genital responses and a
third area of the hypothalamus,
the ventromedial
nucleus, may regulate the expression of
sexual receptivity (lordosis in animals).
Numerous
hypothalamic
994
agents
positively
areas, including
I Essential Psychopharmacology
regulate
estrogen
sexual
motivation
and testosterone,
by their
dopamine,
actions
in these
as well as various
Psychopharmacology
Stage One:
Desire
DA
Stage Two:
Arousal
+
melanocortin
testosterone
estrogen
prolactin
of Sex
NO
+
+
+
+
NE +
melanocortin
testosterone
estrogen
5HT
Stage Three:
Orgasm
ACh
DA
5HT
5HT
NE
DA
NO
+
+
+
+
+
-
+
+/+/-
FIGURE 19-44 Psychopharmacology of sex. The neurotransmitters involved in the three stages of the
psychopharmacology of the human sexual response are summarized here. In stage 1, desire, dopamine (DA),
melanocortin, testosterone, and estrogen exert a positive influence, while prolactin and serotonin (5HT) have
negative effects. In stage 2, arousal correlates with erection in men and genital swelling and lubrication in
women. Several neurotransmitters facilitate sexual arousal, including nitric oxide (NO), norepinephrine (NE),
melanocortin, testosterone, estrogen, acetylcholine (ACh), and DA. As with desire, 5HT has a negative effect.
Stage 3, orgasm, which is associated with ejaculation in men, is inhibited by 5HT and facilitated by NE; DAand
NO may have weak positive influences.
peptide
neurotransmitters
such as melanocortin
the other hand, is hypothesized
This is interesting,
prolactin
prolactin
(Figures
to have a negative influence
since there is a generally
reciprocal
19-44
and
19-45). Prolactin, on
19-44).
on sexual desire (Figure
relationship
between
dopamine
and
(as discussed in Chapter 9; see Figure 9-11). However, the relationship between
and sexual dysfunction is not well documented
and relatively poorly understood.
Serotonin also has a negative influence
to its inhibitory influence on dopamine
dopamine
release is discussed
on sexual motivation
and desire, presumably
due
release (Figure 19-44). Serotonergic
inhibition of
in Chapter 10 and illustrated in Figures 10-21 to 10-25.
Arousal
The second psychopharmacological
stage of the sexual response is arousal (Figure 19-44):
arousal of peripheral tissues, that is. In men, that means an erection; in women, that means
genital lubrication and swelling. This type of arousal prepares the genitalia for penetration
and sexual intercourse. The message of arousal starts in the brain; it is then relayed down
the spinal cord and into peripheral autonomic
parasympathetic;
next, it travels into vascular
nerve fibers that are both sympathetic
and
tissues and finally to the genitalia. Along
Disorders of Reward, Drug Abuse, and Their Treatment
I 995
Sexual Desire and Reward Circuits
nucleus
accumbens
VTA
FIGURE 19-45
Sexual desire and reward circuits. Shown here is the reactive reward system consisting of the
ventral tegmental area (VTA), site of dopamine cell bodies which receives many neurotransmitter projections; the
nucleus accumbens, to which dopaminergic neurons project; and the amygdala (far left), which has connections
with both the VTA and the nucleus accumbens. Dopamine activity in reward circuitry is thought to playa central
role in sexual desire. Dopaminergic neurons also project to the hypothalamus, where they may have input to the
regulation of sexual desire via neurons in the medial preoptic area (MPOA) and the projections of those neurons
to the nucleus accumbens.
the way, at least two key neurotransmitters are involved, acetylcholine in the autonomic
parasympathetic innervation of the genitalia and nitric oxide, which acts upon the smooth
muscle of the genitalia (Figures 19-44 and 19-45). Acetylcholine and nitric oxide both
promote erections in men and lubrication and swelling in women.
Nitric oxide psychopharmacology
Nitric oxide (NO), a gas, is an improbable compound for a neurotransmitter. It is not an
amine, amino acid, or peptide; it is not stored in synaptic vesicles or released by exocytosis;
and it does not interact with specific receptor subtypes in neuronal membranes; but it is
996
I
Essential Psychopharmacology
19-46 Sexual arousal and
neurotransmitters.Sexualarousal in
peripheralgenitaliais accompaniedby
erectionsin men and lubricationand
swellingin women. Bothnitricoxide
and acetylcholineare regulatorsof these
actions.
FIGURE
Sexual Arousal and Neurotransmitters
00
ACh
0 o®
lubrication,
NO
~
-------9d .
swelling
~
erection
"no laughing matter." Specifically, it is not nitrous oxide (N20) or "laughing gas," one of
the earliest known anesthetics. Nitric oxide is a far different gas, although the two are often
confused. It is NO that is the neurotransmitter, not N20. Incredible as it may seem, NO
is a poisonous and unstable gas, a component of car fumes that helps to deplete the ozone
layer, yet is also a chemical messenger both in the brain and in blood vessels, including
those that control erections of the penis.
Yes, there is NO synthesis by neurons and the penis. Certain neurons and tissues possess
the enzyme nitric oxide synthetase (NOS), which forms NO from the amino acid I-arginine
(Figure 19-47 A). NO then diffuses to adjacent neurons or smooth muscle and provokes the
formation of the second messenger cyclic GMP (guanosine monophosphate) by activating
the enzyme guanylyl cyclase (GC) (Figure 19-47B). NO is not made in advance, nor is it
stored; it seems to be made on demand and released by simple diffusion. Glutamate and
calcium can trigger the formation of NO by activating NOS.
No, there are no NO receptors. In striking contrast to classic neurotransmitters, which
have numerous types and subtypes of membrane receptors on neurons, there are no NO
membrane receptors. Rather, the target of NO is iron in the active site of GC (Figure
19-47B). Once NO binds to the iron, GC is activated and cGMP is formed. The action
of cGMP is terminated by a family of enzymes known as phosphodiesterases (PDE), of
which there are several forms, depending upon the tissue (Figure 19-47C).
Yes, there is NO neurotransmitter function. The first known messenger functions
for NO were described in blood vessels. By relaxing smooth muscles in blood vessels of
the penis, NO can regulate penile erections, allowing blood to flow into the penis. NO
also can modulate vascular smooth muscle in cardiac blood vessels and mediate the ability of nitroglycerin to treat cardiac angina. NO is also a key regulator of blood pressure,
platelet aggregation, and peristalsis. Its CNS neurotransmitter function remains elusive,
but it may be a "retrograde neurotransmitter." That is, since presynaptic neurotransmitters activate postsynaptic receptors, it seems logical that communication in this direction
should be accompanied by some form of back talk from the postsynaptic site to the presynaptic neuron. The idea is that NO formation is prompted in postsynaptic synapses by
some presynaptic neurotransmitters and then diffuses back to the presynaptic neuron, carrying information in reverse. NO may also be involved in memory formation, neuronal
plasticity, and neurotoxicity. The notion of retrograde neurotransmission is introduced in
Chapter 3, and the role of NO as a potential retrograde neurotransmitter is illustrated in
Figure 3-3.
Other neurotransmitters and hormones that affect arousal positively include norepinephrine, melanocortin, testosterone, and estrogen (Figure 19-44). Serotonin has a negative influence on sexual arousal (Figure 19-44).
Disordersof Reward,DrugAbuse,and TheirTreatment
997
Nitric Oxide (NO) and Sexual Arousal
A. synthesis of NO
~
L-arginine
~~
~
00
~
0·00
o 0
o
o
o
o
nitric oxide synthetase
o
00
NO (nitric oxide)
0
00
o En:>
000
B. synthesis of cGM P with sexual arousal
~
~J';
00
0
HEME
~
()
lubrication,
'"+'
swelling
erection
A. /'"
_ri
CG~
C. enzymatic destruction of cGMP with loss of sexual arousal
cGMP
~
FIGURE 19-47A,
8, and C Nitric oxide (NO) and sexual arousal. (A) NO is formed by the enzyme nitric oxide
synthetase (NOS), which converts the amino acid I-arginine into NO and I-citrulline. (8) Once formed, NO
activates the enzyme guanylyl cyclase (GC) by binding to iron (heme) in the active site of this enzyme. When
activated, GC makes a messenger, cGMP (cyclic guanylate monophosphate), which relaxes smooth muscle and
performs other physiological functions. In the penis, relaxation of vascular smooth muscle opens blood flow and
causes an erection. (C) The action of cGMP is terminated by the enzyme phosphodiesterase, thus ending sexual
arousal. In the penis, the type of phosphodiesterase is type V (PDE V).
998
Essential Psychopharmacology
Neurotransmitters
serotonin
~~
•••
and Orgasm
9
d
orgasm
ejaculation
and orgasm
"
h"nne .•.•.e}.4:,
noreplnep
4:,
FIGURE 19-48
Neurotransmitters and orgasm. Orgasm is the third stage of the human sexual response,
accompanied by ejaculation in men. Serotonin exerts an inhibitory action on orgasm and norepinephrine a
facilitatoryone.
Orgasm
The third stage of the human sexual response is orgasm (Figure 14-44), accompanied by
ejaculation in men. Descending spinal serotonergic fibers exert inhibitory actions on orgasm
via 5HT receptors, perhaps 5HT2A and 5HT2C receptors (Figure 19-48). Descending
spinal noradrenergic fibers and noradrenergic sympathetic innervation of genitalia facilitate ejaculation and orgasm (Figure 19-44). Dopamine and NO may have weak positive
influences on facilitating orgasm (Figure 19-44).
Sexual disorders and reward
Erectile dysfunction
The inability to maintain an erection sufficient for intercourse is called erectile dysfunction
(formerly impotence). Up to 20 million men in the United States have this problem to
some degree. Another way of stating the problem is that for normal men between 40 and
70 years of age living in the community, only about half do not have some degree of erectile
dysfunction (Figure 19-49). The problem gets worse with age (Figure 19-50), since 39
percent of 40-year-olds have some degree of erectile dysfunction (5 percent are completely
impotent); but by age 70, two thirds have some degree of erectile dysfunction (and complete
impotence triples to 15 percent). The multiple causes of erectile dysfunction include vascular insufficiency, various neurological conditions, endocrine pathology (especially diabetes
mellitus but also reproductive hormones and thyroid problems), drugs, local pathology in
the penis, and psychological and psychiatric problems.
Until recently, psychopharmacologists were not very useful members of the treatment
team for patients with erectile dysfunction other than to stop the medications they were
prescribing! Effective treatment of "organic" causes of erectile dysfunction until recently was
often elusive and usually involved a urological approach, such as prostheses and implants.
Disorders of Reward, Drug Abuse, and Their Treatment
I
999
Prevalence of Erectile Dysfunction
Massachusetts Male Aging Study
men aged 40 to 70 years
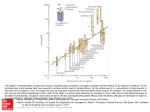
FIGURE 19-49 Prevalence of erectile dysfunction. About half of men between the ages of 40 and 70
experience some degree of erectile dysfunction (impotence).
Association between Age and Prevalence of Erectile Dysfunction (ED)
Massachusetts Male Aging Study
--.
§!
*
C
Q
~
c:
'-'
.9!
QJ
80
40
67%
20
57%
600
48%
39%
•
40
50
60
•
70
age (y)
D
complete ED ~
D
moderate ED~
III minimal ED
FIGURE 19-50
•
Association between age and prevalence of erectile dysfunction
(ED). In this study of normal
men between the ages of 40 and 70, the prevalence of erectile dysfunction increased with age from 39 percent
at age 40 to 67 percent at age 70.
1000
I
Essential Psychopharmacology
Association between Depression and Prevalence of Erectile Dysfunction
Massachusetts Male Aging Study
90%
100
-6'
OJ
c
C
:c
()
O
JJ
.Q
u;
(tj
OJ
Q;
t5
t5
5;
a.
'0
,g
80
40
60
~20
o
mild
depression
moderate
depression
severe
depression
FIGURE 19-51 Associationbetween depressionand prevalenceof erectiledysfunction (ED). Erectile
dysfunctionis associatedwith depressionand increasesin frequencyas depressionworsens.Some studies
suggestthat over 90 percentof severelydepressed men have erectiledysfunction.
The old-fashioned surgical strategy bypasses diseased peripheral nerves and inadequate
vascular blood supply to the penis to create erections mechanically and upon demand, but
it has serious limitations in terms of patient and partner acceptability. In men who have a
"functional" etiology to their erectile dysfunction, the treatment strategy has traditionally
taken a psychodynamic and behavioral approach, with attention to partners and functional
disorders, psychoeducation, lifestyle changes, and, where appropriate, starting (or stopping)
psychotropic drugs to treat associated disorders. The typical case of erectile dysfunction,
however, is due to neither a single "organic" cause nor a single "functional" cause but
usually involves some combination of problems, including use of alcohol, smoking, diabetes,
hypertension, antihypertensive drugs, psychotropic drugs, partner problems, performance
anxiety, problems with self-esteem, and psychiatric disorder, especially depression.
The topic of erectile dysfunction has become increasingly important in psychopharmacology, not only because there are several psychotropic drugs that cause it but also because
of the strikingly high incidence of erectile dysfunction in several common psychiatric disorders. For example, some studies show that more than 90 percent of men with severe
depression have moderate to severe erectile dysfunction (Figure 19-51). Another reason
for the importance of this topic in psychopharmacology is that several effective and simple
psychopharmacological treatments based upon NO physiology and pharmacology are now
available for men with erectile dysfunction.
Disordersof Reward,DrugAbuse,and TheirTreatment I 1001
Normal Erectile Function
o
o
o
o
o
®
0
® ®
0 \
o 0 @
@ 0
FIGURE 19-52
Normal erectile function.
'l
Under normal conditions, when young, healthy men are sexually
aroused, nitric oxide causes cGMP to accumulate, and cGMP causes smooth muscle relaxation, resulting in a
physiological erection (indicated here by an inflated balloon). The erection is sustained long enough for sexual
intercourse, and then phosphodiesterase V (PDE V) metabolizes cGMp, reversing the erection (indicated here by a
pin ready to prick the balloon).
Psychopharmacology of erectile dysfunction
Normally, the desire to have sex is a powerful message sent from the brain down the
spinal cord and through peripheral nerves to smooth muscle cells in the penis, triggering
them to produce plenty of NO to form all the cyclic GMP necessary to create an erection
(Figure 19-52). The cyclic GMP lasts long enough for sexual intercourse to occur, but then
phosphodiesterase (type V in the penis) eventually breaks down the cGMP (shown earlier
in Figure 19-47C) and the erection is lost (called detumescence).
However, if you smoke, eat to the point of obesity, have elevated blood glucose and
elevated blood pressure, your peripheral nervous system "wires" may not respond adequately
to the "let's have sex" signal from the brain (i.e., neurological innervation of the penis
is rendered faulty, usually by diabetes) (Figure 19-53). Furthermore, there may not be
much pressure in the plumbing (i.e., atherosclerosis of the arterial supply of the penis from
hypertension and hypercholesterolemia) when cGMP says "relax the smooth muscle and
let the blood flow into the penis." In these cases, the desire to have sex is there, but the
signal cannot get through, so insufficient cGMP is formed and therefore no erection occurs
(Figure 19-53). Similarly, for a depressed patient who has the desire for sex, there is a
general shutdown of neurotransmitter systems centrally and peripherally and the inability
to become aroused (Figure 19-53).
Fortunately, there is a way to compensate for inadequate amounts of cGMP being
formed. That compensation is to slow the rate of destruction of that cGMP that is formed.
This is done by inhibiting the enzyme that normally breaks down cGMP in the penis, namely
phosphodiesterase type V (Figure 19-54). There are now three inhibitors of this enzyme
available: sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra) (Figure 19-54).
These phosphodiesterase type V inhibitors will stop cGMP destruction for a time ranging
from a few hours to a few days and allow the levels of cGMP to build up; therefore
1002
Essential Psychopharmacology
Erectile Dysfunction (ED)
o
o
o
o
o
@0
® ®
0 \~
o 0
@
FIGURE 19-53
0
'l@
Erectile dysfunction. When a man has diabetes or hypertension or if he smokes, uses alcohol,
takes prescription drugs, or is depressed, there is a good chance that not enough of a signal of sexual desire will
be able to get through his peripheral nerves and arteries to produce sufficient amounts of cGMP to cause an
erection. This leads to erectile dysfunction.
Treatment of Erectile Dysfunction (ED)
o
o
o
o
o
®
0
® ®
FIGURE 19-54
0 ®\~
o 0
0
®
'l
Treatment of erectile dysfunction. Phosphodiesterase V (PDE V) inhibitors are able to
compensate for faulty signals through the peripheral nerves and arteries that produce insufficient amounts of
cGMP to cause or sustain an erection. PDE V inhibitors do this by allowing cGMP to build up, since PDE V can no
longer destroy cGMP for a few hours to a few days. This is indicated by a patch on the balloon in the figure. The
result is that normally inadequate nerves and arteries signaling cGMP formation are now sufficient to inflate the
balloon and therefore an erection can occur and sexual intercourse is possible until the drug wears off a few hours
to a few days later.
Disorders of Reward, Drug Abuse, and Their Treatment
1003
an erection can occur even though the "wires" and "plumbing" are still faulty (Figure 19-53).
Interestingly, these agents work only if the patient is mentally interested in sex and attempts
to become aroused, so that at least weak signals are sent to the penis (i.e., it does not work
during sleep).
Smooth muscle relaxation is thus the key element in attaining an erection. Administration of prostaglandins can also relax penile smooth muscle and elicit erections in a
manner that mimics typical physiological mechanisms. Thus, intrapenile injection of the
prostaglandin alprostadil produces erections not only in men with organic causes of impotence but also in those with functional causes and even in the common situation of multifactorial causes. Limitations of this somewhat masochistic approach include the unacceptability
of self-injection, lack of spontaneity, and the possibility of too much of a good thing, namely
a prolonged and painful erection, or priapism. Prostaglandin administration will cause an
erection whether the man is mentally aroused or not.
Other drugs can affect sexual arousal, including SSRIs or SNRIs in some patients.
Some of these agents may inhibit NOS directly and thus can cause erectile dysfunction.
On the other hand, some dopaminergic agents might boost NOS, and for this reason
pro-dopaminergic agents may be useful not only in enhancing desire but also in enhancing arousal. Anticholinergic agents can interfere directly with arousal and cause erectile
dysfunction. Thus, those antipsychotics, tricyclic antidepressants, and other drugs with
anticholinergic properties can cause erectile dysfunction.
Hypoactive sexual desire disorder (HSDD)
Another disorder of sexual function is decreased libido, lack of sexual motivation, and
decreased sexual fantasies, known as hypoactive sexual desire disorder (HSDD). This is a
controversial concept, because it can be difficult to separate a chronic and disabling condition
from common transient alterations in sexual behavior related to interpersonal problems, life
stress, and just common fatigue, overwork, and sleep deprivation that are part of living in
the modern world. Now that there are potential treatments on the horizon, is it possible that
HSDD is just a "corporate sponsored creation of a new disease"? Furthermore, decreased
libido is often part of major depressive disorder (see discussions in Chapters 11 and 12 and
Figures 11-45, 11-55, and 12-124 through 12-126), so is it possible that decreased libido
is more likely to be a residual symptom of a major depressive episode that has not gone
into remission rather than an independent clinical entity (Figure 19-55)? Finally, HSDD
is often considered to be mostly a disorder of females, perhaps especially by men, so is it a
gender relationship issue of men rather than a true form of sexual dysfunction in women?
Many women report reduction of sexual desire with the duration of a relationship, and
some may have lost interest in their partner but may still feel like having sex with the
guy next door. Such issues are not likely to be resolved by taking drugs to enhance sexual
desire, and answers to these various questions may be forthcoming as further research is
done on the symptom of decreased libido. Nevertheless, it is already clear that certain
hormones and drugs can increase sexual desire in those who complain of having too little
of it.
Like many of the disorders discussed in this text, to the extent that the core symptoms
of decreased libido and decreased sexual fantasies define HSDD, there are frequently many
associated nondiagnostic symptoms that are equally important to assess and treat (Figure
19-55). These include various symptoms of major depression, not just depressed mood and
decreased libido itself but also change in appetite and weight (self-image and body-image
problems can interfere secondarily with sexual desire); apathy, loss of interest and lack of
1004 I
Essential Psychopharmacology
vasomotor
symptoms
FIGURE 19-55 Hypoactive sexual desire disorder (HSDD). HSDD is a disorder of sexual function characterized
by decreased libido and decreased sexual fantasies. In addition, there are many associated nondiagnostic
symptoms, as shown here. These include various symptoms of major depression, vasomotor symptoms in
perimenopausal women, hypoestrogen and/or hypotestosterone states in women, and hypotestosterone states in
men.
experiencing pleasure globally and not just with sex; lack of motivation to do many things,
not just sexual intercourse; and of course fatigue, which may be the greatest antiaphrodesiac
known (Figure 19-55). Also, a number of other conditions may be associated with HSDD,
including vasomotor symptoms in perimenopausal women, discussed extensively in Chapter
12 and illustrated in Figures 12-104, 12-106 and 12-124 through 12-126. Hypoestrogen
and hypotestosterone states in women and hypotestosterone states in men can also be
associated with lack of sexual interest as one of the symptoms (Figure 19-55).
Thus the symptom of lack of sexual interest requires in-depth evaluation and then
constructing a proper diagnosis (Figure 19-55). The strategy then is to deconstruct the
various symptoms of the patient's syndrome (e.g., those listed in Figure 19-55) and match
them to hypothetically malfunctioning brain circuits (Figure 19-56). Knowing the neurotransmitters (and hormones) that affect neurotransmission in these circuits provides a
psychopharmacological rationale for selecting and combining treatments to eliminate the
patient's symptoms. Note in Figure 19-56 that the great majority of symptoms associated
with HSDD (shown in Figure 19-55) are linked to the nucleus accumbens, the critical area
of the brain that regulates reward (Figures 19-2, 19-3, and 19-45).
Although there are no approved treatments for HSDD, several approaches targeting
neurotransmitters and hormones in reward circuitry are being tested in clinical trials. The
notion is that the low sexual desire ofHSDD is linked to low functioning of dopaminergic
reward neurons in the mesolimbic pathway (Figure 19-57 A). Indeed, anecdotal observations
document that some patients taking levodopa or dopamine agonists for Parkinson's disease
experience increased sex drive, as do some patients taking the pro-dopaminergic antidepressant and NDRI bupropion. Testosterone may enhance sexual interest by actions on
neurons in the hypothalamus and boost the ability of dopamine to act in the hypothalamus.
Disorders of Reward, Drug Abuse, and Their Treatment
I
1005
Some Key HSDD Symptoms Hypothetically
interests
apathy
pleasure
libido
fatigue
euphoria
reward
motivation
sexual arousal
drug abuse
Linked to Specific Brain Regions
physicalfatigue
depressed mood
estrogen/testosterone disturbances
appetite/weight
vasomotorsymptoms
delayed orgasm/anorgasmia
ejaculatory disturbances
FIGURE 19-56 Matching hypoactive sexual desire disorder (HSDD) symptoms to circuits. Alterations in
neurotransmission within each of the brain regions shown here can be hypothetically linked to the various
symptoms associated with HSDD. Functionality in each brain region may be associated with a different
constellation of symptoms. PFC: prefrontal cortex; BF: basal forebrain; S: striatum; NA: nucleus accumbens; T:
thalamus; HY: hypothalamus; A: amygdala; H: hippocampus; NT: brainstem neurotransmitter centers; SC: spinal
cord; C: cerebellum.
In fact, testosterone
has shown positive results in women given low doses transdermally,
but concerns about long-term
safety led the FDA to withhold approval of this approach
pending more safety studies.
One
novel approach
melanocortin
receptors
to HSDD
is to administer
in the hypothalamus
(Figure
a peptide
19-57B).
lanotide is an agonist at melanocortin
MC3 and MC4
receptors in the hypothalamus
increases sexual behavior
intranasally
Specifically,
that acts on
the drug breme-
receptors, and stimulating
these
in animals, boosts the actions of
DA in hypothalamic
areas such as the medial preoptic area (MPOA) (see Figure 19-57B),
and also shows preliminary
evidence of efficacy in women with HSDD (and also in men
with erectile dysfunction).
raise blood pressure.
Concerns
have arisen, however,
about the ability of this drug to
Another novel approach to HSDD is the serotonergic
agent flibanserin, which has
shown preliminary evidence of efficacy in women with HSDD. Flibanserin
acts as a norepinephrine
of5HT1A
and dopamine disinhibitor
(NDDI)
agonism, plus 5HT2A and 5HT2C
by means of its pharmacological
properties
antagonism (Figure 19-57B). NDDI action
due to 5HT2C antagonism
is discussed extensively in Chapter 12 and illustrated in Figure
12-25. The enhancement
of dopamine release by combining 5HT2C (and 5HT2A) antagonism with agonist actions at 5HT1A receptors is discussed in Chapter 10 and illustrated in
Figure 10-21; it is also discussed in Chapter 12 and illustrated in Figures 12-61 through 1264. Flibanserin increases dopamine (and norepinephrine)
release and also reduces serotonin
release, properties
that would enhance
when these actions occur in the MPOA
1006
Essential Psychopharmacology
reward and hypothetically
and nucleus accumbens
increase sexual motivation
(Figure
19-57B).
Ongoing
HSDD: Low Sexual Desire, Low Dopamine
nucleus
accumbens
"I have a headache"
MPOA
-,1tDA
·
·
··
raphe
5HT1A
:
-..••".
DA
• neuron
VTA
5HT
5HT
",
~t46c'"
GABA
ZI
MPOA = medial pre optic area
ZI = zona incerta
I
normal
. baseline
overactivation
hypoactivation
A
FIGURE 19-57A Hypoactive sexual desire disorder (HSDD): low dopamine? Low sexual desire in HSDD is
believed to be due to hypoactivity of mesolimbic dopaminergic neurons (depicted here by the blue color and
dashed lines of the neuron).
research will determine whether flibanserin has sufficient efficacy and safety in HSDD to
possibly become the first approved treatment for this condition.
Compulsive sexual behavior
A number of conditions linked to sexual activity of various sorts have been categorized as
disorders of impulsivity (Table 19-5). Hypothetically, some of these conditions could be
linked to abnormal activity of reward circuits (Figure 19-2), analogous to an addiction,
where there is deficient descending inhibitory influence from the reflective reward system
in the prefrontal cortex (Figure 19-3) to stop the expression of abnormal sexual drives
arising from the reactive reward system of the VTA and amygdala (Figure 19-4). These
concepts are controversial if novel, and therapeutic approaches targeting neurotransmitters
in reward circuitry (Figures 19-5 through 19-8) may eventually provide relief for some of
these disorders (Table 19-5).
Disorders of Reward, Drug Abuse, and Their Treatment
I 1007
Treatment of HSDD: Raising Dopamine Improves Sexual Desire
nucleus
accumbens
MPOA
raphe
__:'.1
..
5HT "'\
neuron.-
5J:1T1 A
":;
,
c.
VTA
ZI
flibanserin
MPOA = medial pre optic area
ZI = zona incerta
I
normal
baseline
~ overactivation
hypoactivation
B
FIGURE 19-578 Treatment of hypoactive sexual desire disorder (HSDD) by raising dopamine. Potential
treatments for HSDD include agents that increase dopaminergic neurotransmission in the nucleus accumbens.
This may be achieved with bremelanotide, which is an agonist at melanocortin 3 and 4 (MC3 and MC4)
receptors in the medial preoptic area (MPOA) of the hypothalamus, or by flibanserin, which is a 5HTlA agonist
and a 5HT2A and 5HT2C antagonist.
Eating disorders
Eating disorders and reward circuits
Should
obesity be included
as a brain disorder?
Did my receptors
make me eat it? Obesity
is a complex disorder, with lifestyles, diet, and exercise playing major roles in twenty-first
century society. Certainly there is an ongoing epidemic of obesity and metabolic disorder
in society today; this is discussed in Chapter 10 in relation to antipsychotic
drugs and
illustrated in Figures 10-59 through 10-69. Chapter 10 also discusses the potential roles
of genes associated with various mental illnesses and drugs used to treat mental illnesses as
additional
risk factors for obesity, and ultimately
It is also possible
that some forms of obesity
drive for food, mediated
1008
by reward
Essential Psychopharmacology
circuitry,
cardiometabolic
disorders.
are driven by an excessive
and as such might
motivational
be considered
as mental
TABLE 19-5
Psychopharmacologyand sexualdisorder
Erectile dysfunction (ED)
Hypoactive sexual desire disorder (HSDD)
Anorgasmial ejaculatory delay
Premature ejaculation
Sexual pain disorders
SSRI/SNRI -induced sexualdysfunctions
Sexual "addictions"- impulsivity/compulsivitydisorders:
Paraphilias
Exhibitionism
Fetishism
Frotteurism
Pedophilia
Sexual sadism
Sexual masochism
Transvestic fetishism
Voyeurism
Compulsive cruising and multiple partners
Compulsive fixation on an unattainable partner
Compulsive autoeroticism
Compulsive use of erotica
Compulsive use of the internet
Compulsive multiple love relationships
Compulsive sexualityin a relationship
disorders. Can you be addicted to food with compulsive consumption and the inability to
refrain from eating despite the desire to do so? Can you be addicted to carbohydrates or
your "sugar fix?" Are high-fat foods "comfort foods" that relieve craving and cause pleasure
because they are addicting?
These symptoms associated with obesity, overeating, and binge eating in many patients
are remarkably similar to the addictions to various drugs described in this chapter. A hypothetical if oversimplified idea for how certain eating disorders could be linked to reward
circuits is shown in Figure 19-58, with dopamine projections not only to the nucleus accumbens but also to the mammillary nucleus of the hypothalamus, an area of the hypothalamus
that exerts important regulatory control of eating in animals. This region projects to nucleus
accumbens as well, where it may regulate the motivation to eat (and addiction to food?).
Current research is attempting to clarifY the role of a long list of other key regulators of hypothalamic activity in eating: leptin, ghrelin, anandamide, neurotensin, CRF,
cholecystokinin, insulin, glucagon, calcitonin, amylin, bonbesin, somatostatin, cytokines,
melanocortin, orexin, dynorphin, beta endorphin, galanin, neuropeptide Y and many other
hormones, neurotransmitters and hypothalamic peptides. The roles of hypothalamic serotonin actions, particularly at 5HT2C receptors, and hypothalamic histamine actions, particularly at HI receptors, are discussed in Chapter 10 and illustrated in Figure 10-59.
Treatments for compulsive
eating, obesity, and "food addiction"
The prevalence and morbidity of obesity are sufficiently vast that it is important if not
urgent to develop therapeutic interventions. It is possible that some patients could benefit
from approaches that target hypothalamic and mesolimbic reward circuits, since obesity
Disordersof Reward,DrugAbuse,and TheirTreatment I 1009
Eating, Hunger, and Reward Circuits
nucleus
accumbens
VTA
FIGURE 19-58
Eating, hunger, and reward circuits. Shown here is the reactive reward system consisting of the
ventral tegmental area (VTA) , site of dopamine cell bodies that receives many neurotransmitter projections; the
nucleus accumbens, to which dopaminergic neurons project; and the amygdala (far left), which has connections
with both the VTA and the nucleus accumbens. In addition, dopamine projections extend to the mammillary
nucleus (MAM) of the hypothalamus, an area important for regulatory control of eating; projections from these
regions themselves extend to the nucleus accumbens. Thus the circuitry of hunger is interconnected with the
circuitry for reward.
may not always be just a metabolic disorder but, in some cases, a disorder of reward circuits
or even an addiction.
Current drugs of abuse, especially stimulants and nicotine, reduce appetite. Others,
such as marijuana, actually stimulate appetite, leading to the use of the cannabinoid CBl
antagonist for the treatment of obesity. As mentioned earlier, this agent is approved in
some countries but not in the United States. Approved stimulants for obesity, including
the SNRI sibutramine, have fallen into relative disrepute given their lack of long-term
efficacy in most patients plus the risk of hypertension and the toxicity scare caused by the
amphetamine derivative fenfluramine in the recent past. Orlistat, now available without
prescription, inhibits fat absorption and works peripherally and not on reward circuitry
1010
I
Essential Psychopharmacology
except to the extent that it causes an aversive response to eating fatty foods (diarrhea and
flatus). It is not highly utilized or highly palatable to many patients.
Fluoxetine is approved for bulimia and acts perhaps in part to suppress appetite via
its 5HT2C antagonist properties (see Figure 12-25). Other agents active at 5HT2C sites,
including both agonists (e.g., GSK 875167 and others) and antagonists (see the list at the
end of Chapter 10 on new treatments for schizophrenia) are also in testing for obesity.
The anticonvulsants topiramate and zonisamide and the ADHD drug and norepinephrine reuptake inhibitor atomoxetine have anecdotally been associated with weight
loss in some patients and are now in testing for obesity. Some medications approved for diabetes may hold promise for the treatment of obesity, including metformin and the injectable
peptide pramlintide (Symlin; discussed in Chapter 10). Another novel agent is Axokine, or
ciliary neurotrophic factor, which is in testing for obesity, since it seems to cause weight loss
in humans. Cholecystolinin agonists (e.g., GSK 181771) and other agents active at various peptide receptors, including bremelanotide (MC3 and MC4 agonist discussed above
for treatment of HSDD and illustrated in Figure 19-57B) are also in testing for obesity.
Some analysts estimate that there are actually hundreds of agents in testing for obesity in
the hope that some therapeutic intervention can be found for this epidemic. Some of the
approaches target reward circuitry (Figure 12-58) and approach obesity as a disorder of
reward, analogous to an addiction.
Other impulse-control
disorders
A number of other conditions have been linked to reward circuitry and have been conceptualized as addictions. This includes borderline personality disorder, compulsive gambling,
kleptomania, pyromania, compulsive shopping, and other related conditions. Whether these
will prove to be disorders of reward and whether they will prove treatable by approaching
them as addictions remains a topic of intense current interest and research.
Summary
This chapter discussed the psychopharmacology of reward and the brain circuitry that regulates reward. We have attempted to explain the psychopharmacological mechanisms of
action of various drugs of abuse, from nicotine to alcohol, and also opiates, stimulants,
sedative hypnotics, marijuana, hallucinogens, and club drugs. In the case of nicotine and
alcohol, various novel psychopharmacological treatments are discussed, including the alpha
4 beta 2 selective nicotine partial agonist (NPA) varenicline for smoking cessation and
naltrexone and acamprosate for alcohol dependence. For each of the drug classes explored,
their hypothetical actions upon mesolimbic reward circuitry are explained. Disorders of
sexual function hypothetically linked to dysregulation of reward mechanisms in this same
circuitry are also explored. Finally, a number of disorders are discussed that are not recognized substance abuse disorders but may be forms of addiction, including obesity, gambling,
and other related conditions.
Disorders of Reward, Drug Abuse, and Their Treatment
I 1011




































![NALTREXONE[1].](http://s1.studyres.com/store/data/008499817_1-96b3e8696c43dc1d94e990e6680b7eac-150x150.png)