* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download signal molecule
Neurotransmitter wikipedia , lookup
Synaptogenesis wikipedia , lookup
NMDA receptor wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
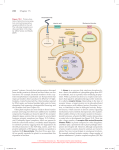
Cell Communication Signaling molecules & Cell surface receptors Cell Communication: An Overview Cells communicate with one another through Direct channels of communication Specific contact between cells Intercellular chemical messengers Receptor animation Cell surface receptors Cell Communication To survive, cells must Communicate with their neighbors Monitor environmental conditions Respond appropriately Cell Signaling Apoptosis Fig. 7-1, p. 140 Signals relayed between cells Direct intercellular signaling 1. Cell junctions allow signaling molecules to pass from one cell to another Direct Contact-dependent signaling (Juxtacrine) 2. Some molecules are bound to the surface of cells and serve as signals to cell coming in contact with them Autocrine signaling 3. Cells secrete signaling molecules that bind to their own cell surface or neighboring cells of the same type 8 Signals relayed between cells Paracrine signaling 3. Signal does not affect cell secreting the signal but does influence cells in close proximity (synaptic signaling) Endocrine signaling 4. Signals (hormones) travel long distances and are usually longer lasting 9 Synaptic signaling is similar to paracrine signaling but there is a special structure called the synapse between the cell originating and the cell receiving the signal. Synaptic signaling only occurs between cells with the synapse; for example between a neuron and the muscle that is controlled by neural activity. 11 Cell Signaling Signaling Molecules Small molecules Peptides Proteins LIGANDS Receptor affinity High Low concentration of ligand; most receptors are occupied Low affinity affinity High concentration of ligand for most rectors to be occupied Receptor affinity Dissociation constant Kd Measures the affinity of the receptor-ligand complex The concentration of ligand at which half the receptors are occupied Example Erythroid progenitor cell ~1000 surface receptors for erythropoietin (Epo) Only 100 receptors need to bind Epo to induce cell division Max cellular response less than Kd Vasoconstriction occurs when epinephrine (adrenaline) binds to the a-adrenergic receptor on vascular smooth muscle cells. One approach to treating high blood pressures is to administer competitive inhibitors that bind to the a-adrenergic receptor. The Kd for binding of epinephrine to this receptor is ~0.6 mM. Which of the following compounds might be good candidate drugs for high blood pressure? Kd for binding to the a-adrenergic 33% receptor are shown Compound A: Kd = 1pM 2. Compound B: Kd = 0.6 mM 3. Compound C: Kd = 60 mM . 33% 33% 1. 1 2 3 Intercellular Chemical Messengers Controlling Releases signal molecule that causes response of target cells Target cell processes signal in 3 steps: Reception, transduction, response Signal cell transduction Series of events from reception to response 3 stages of cell signaling Receptor activation 1. Signaling molecule binds to receptor Signal transduction 2. Activated receptor stimulates sequence of changes- signal transduction pathway Cellular response 3. Several different responses • • • Alter activity of 1 or more enzymes Alter structural protein function Change gene expression– transcription factor 22 Signal Transduction Fig. 7-2, p. 142 24 Amazing cells animation Which of the following best describes a signal transduction pathway? Binding of a signal molecule to a cell protein 2. Catalysis mediated by an enzyme 3. Series of changes in a series of molecules resulting in a response 1. 33% 1 33% 2 33% 3 a. Reception by a cell-surface receptor Polar (hydrophilic) signal molecule Activation Receptor embedded in plasma membrane Target cell Plasma membrane Polar signal molecules cannot pass through the plasma membrane. In this case they bind to a receptor on the surface. Fig. 7-3a, p. 142 b. Reception by a receptor within cell Nonpolar (hydrophobic) signal molecule Activation Receptor within cell Nonpolar signal molecules pass through the plasma membrane and bind to their receptors in the cell. Fig. 7-3b, p. 142 Intracellular receptors Some receptors are inside the cell Estrogen example Passes through membrane and binds to receptor in nucleus Dimer of estrogen•receptor complexes binds to DNA • Transcription factors regulate transcription of specific genes 29 30 Cell Communication Systems with Surface Receptors Peptide Primary extracellular signal molecules recognized by surface receptors in animals Surface hormones and neurotransmitters receptors Integral membrane glycoproteins Signaling molecule Bound by a surface receptor Triggers response pathways within the cell Surface Receptors Cell communication systems based on surface receptors have 3 components: (1) Extracellular signal molecules (2) Surface receptors that receive signals (3) Internal response pathways triggered when receptors bind a signal Peptide Hormones Peptide Small proteins Growth hormones factors Special class of peptide hormones Affect cell growth, division, differentiation Neurotransmitters Neurotransmitters include Small peptides Individual amino acids or their derivatives Chemical substances Surface Receptors Surface Integral membrane proteins Extend entirely through the plasma membrane Binding receptors of a signal molecule Induces molecular change in the receptor that activates its cytoplasmic end Ligand Signaling molecule Binds noncovalently to receptor with high degree of specificity Binding and release between receptor and ligand relatively rapid Ligands alter receptor structureconformational change Once a ligand is released, the receptor is no longer activated 36 Response of Surface Receptor Fig. 7-4, p. 143 38 Cellular Response Pathways Cellular Operate by activating protein kinases Protein response pathways kinases add phosphate groups Stimulate or inhibit activities of target proteins, producing cellular response 40 Cellular Response Pathways Protein phosphatases Reverse response Remove phosphate groups from target proteins Receptors are removed by endocytosis When signal transduction is finished Phosphorylation Fig. 7-5, p. 144 Amplification Each step of a response pathway catalyzed by an enzyme is amplified Each enzyme activates hundreds or thousands of proteins that enter next step in pathway Amplification Allows full cellular response when few signal molecules bind to receptors Amplification Fig. 7-6, p. 145 45 Which of the following steps in an intracellular signaling pathway amplifies the signal? 1. Synthesis of a 25% 25% 25% 25% secondary messenger 2. Activation of a protein kinase 3. Binding of ligand to receptor 4. 1 & 2 1 2 3 4 In reactions mediated by protein kinases, what does phosphorylation of successive proteins do to drive the reaction? 1. 2. 3. Make functional ATP Change a protein from its inactive to active form Change a protein from its active to inactive form 33% 1 33% 2 33% 3 Which of the following is an example of signal amplification? 1. 2. 3. catalysis of many cAMP molecules by several simultaneously binding signal molecules activation of 100 molecules by a single signal binding event activation of a specific gene by a growth factor 33% 1 33% 2 33% 3 Cell surface receptors Enzyme-linked receptors 1. Found in all living species Extracellular domain binds signal Causes intracellular domain to become functional catalyst Most are protein kinases 50 Receptor Tyrosine Kinases Receptor tyrosine kinases bind signal molecule Protein kinase site becomes active Adds phosphate groups to tyrosines in the receptor itself, and to target proteins Phosphate groups added to cytoplasmic end of receptor are recognition sites for proteins activated by binding to the receptor Protein Kinase Activity Fig. 7-7, p. 146 Acetylcholine mimics the electrical stimulation of the vagus nerve. It is now known to be a neurotransmitter at all autonomic ganglia, at many autonomically innervated organs, at the neuromuscular junction, and at many synapses in the CNS. What nerve! https://www.khanacademy.org/science/biol ogy/human-biology/neuron-nervoussystem/v/anatomy-of-a-neuron http://www.biologymad.com/nervoussyste m/nerveimpulses.htm Nerves are either “open” or “closed” The neurotransmitter is broken down by a specific enzyme in the synaptic cleft; for example the enzyme acetylcholinesterase breaks down the neurotransmitteracetylcholine. The breakdown products are absorbed by the pre-synaptic neurone by endocytosis and used to re-synthesise more neurotransmitter, using energy from the mitochondria. This stops the synapse being permanently on. Nerve agents Organochlorine compounds work on insects by opening what's known as the sodium ion channel in the neurons or nerve cells of insects, causing them to fire spontaneously. The insect will go into spasms and eventually die. DDT was the earliest of these chlorinated hydrocarbons. Note: There are many other classes of insecticides….this is just the one that effects the “twitching” Birth Control Pills “fool” the Body The lower levels of estrogen in birth control pills suppress FSH (Follicle Stimulating Hormone) and LH (luteinizing hormone) "fooling" the pituitary gland into thinking a woman is pregnant. Ovulation will then not occur, which prevents pregnancy. Go to next slide.. The progesterone in birth control pills creates a thick cervical mucus, making it difficult for sperm to reach theuterus. It also impedes an egg from attaching itself to the uterine lining (endometrium) because of changes in the cellular structure of the lining. G-Protein–Coupled Receptors G proteins: Key molecular switches in second-messenger pathways Two major G-protein–coupled receptor response pathways involve different second messengers G-Protein-Coupled Receptors G-protein-coupled receptors activate pathways Binding of the extracellular signal molecule (first messenger) activates a site on the cytoplasmic end of the receptor G-protein-coupled receptors Signals binding to cell surface are first messenger Many signal transduction pathways lead to production of second messengers Relay signals inside cells Examples • cAMP • Ca2+ • Diacylglycerol and inositol triphosphate 63 G-Protein-Coupled Receptors Fig. 7-8, p. 147 Disruption of G Protein signaling causes several human diseases. Vibrio cholerae (causes cholera) secretes the cholera toxin which alters salt and fluid in the intestine normally controlled by hormones that activate Gs G-Protein to increase cAMP. The cholera toxin enzymatically changes Gs so that it is unable to convert GTP to GDP. Gs can not then be inactivated and cAMP levels remain high causing intestinal cell to secrete salt and water. Eventually dehydration can lead to death (cholera). Now what? How does binding a signaling molecule induce a cellular response? 68 G-protein-linkedreceptors 7-pass transmembrane receptor + G protein G protein = GTP binding protein G proteins are trimeric = 3 subunits Inactive State Receptor binds ligand G-protein associates with receptor GTP is exchanged for GDP - a subunit and subunit activated The G-protein a-subunit and subunits are activated What next? The active subunits interact with target proteins in the membrane What are some target proteins? G-Protein Activation Activated receptor turns on a G protein, which acts as a molecular switch G protein Active when bound to GTP Inactive when bound to GDP Active G Protein Active G protein Switches on the effector of the pathway (enzyme that generates second messengers) Second messengers Small internal signal molecules Activate the protein kinases of the pathway Response Pathways Fig. 7-9, p. 147 Second Messengers: cAMP 1st of two major pathways triggered by Gprotein-coupled receptors Effector (adenylyl cyclase) generates cAMP as second messenger cAMP activates specific protein kinases cAMP Receptor-Response Pathways Fig. 7-10, p. 148 cAMP Fig. 7-11, p. 148 Adenylyl cyclase Phosphodiesterase Pyrophosphate ATP cAMP (second messenger) AMP Fig. 7-11, p. 148 81 One effect of cAMP is to activate protein kinase A (PKA) Activated catalytic PKA subunits phosphorylates specific cellular proteins When signaling molecules no longer produced, eventually effects of PKA reversed 82 83 cAMP has 2 advantages 1. Signal amplification 2. Binding of signal to single receptor can cause the synthesis of many cAMP that activate PKA, each PKA can phosphorylate many proteins Speed In one experiment a substantial amount of cAMP was made within 20 seconds after addition of signal 84 Now what? How does binding a signaling molecule induce a cellular response? Membrane-bound Enzymes Second messengers Adenylate cyclase Adenylate Cyclase a cAMP Adenylyl cyclase Always “on” so cAMP is quickly broken down cAMP activates cAMP-dependent protein kinase (A-kinase) A-kinase phophorylates serine/threonines of selected proteins Regulates the activity of the target protein Activated A-kinase can modulate gene regulation Example of cell regulation by an increase in cAMP levels Fight or flight response- muscle cells Fight or flight response When an animal is frightened or stressed the adrenal gland releases epinephrine into the bloodstream Epinephrine example Fight-or-flight hormone Different effects throughout body Stimulates heart muscle cells to beat faster Caffeine inhibits phosphodiesterase Enzyme removes cAMP once a signaling molecule is no longer present Inhibition causes cAMP to persist for longer so heart beats faster 95 96 -adrenergic receptors Circulating epinephrine binds -adrenergic receptors on muscle and liver cells Liberates glucose and fatty acids animation A-kinase phosphoryates an enzyme to break down glycogen to release glucose 99 β- adrenergic receptors β- adrenergic receptors are GPCR Different types are coupled to different G proteins Gs (stimulatory) G proteins activate adenylyl clyclase Gi (inhibitory) G proteins inhibit adenylyl cyclase (α1 and α2) Phophoprotein phosphatase (PP) Pathway Controls cAMP pathways are balanced by reactions that eliminate second messengers Stopped by protein phosphatases that continually remove phosphate groups from target proteins Stopped by endocytosis of receptors and their bound extracellular signals 103 Grb2SH2 adaptor protein Sos Guanine nucleotide exchange factor Ras-GEF 1. Receptor binds ligand 2. Tyrosines phosphorylated 4. Sos exchanges GTP for GDP 3. Grb2/Sos bind pY Activated Ras recruits Raf to the plasma membrane Raf - protein kinase that initiates the MAP kinase cascade Ras and Gα (trimeric G proteins) Similar structure and function and ubiquity in eukaryotic cells suggest a single type of GTPase originated early in evolution Gene encoding this ancestral protein duplicated and evolved > 100 different intracellular switch proteins Active GTP Ras-GTP active conformation Ras-mitogen-activated protein kinase (MAPK) Similar to cAMP signaling cascade - both provide pathways by which extracellular signals can influence gene expression Cascade of Protein Kinases Active Ras activates Raf (ser/thr kinase) Raf activates MEK MEK activates MAPK MAPK activates other proteins (transcription factors) Gene Regulation: Ras Some pathways in gene regulation link certain receptor tyrosine kinases to a specific G protein (Ras) When the receptor binds a signal molecule, it phosphorylates itself Adapter proteins then bind, bridging to and activating Ras Activated Ras Activated Ras turns on the MAP kinase cascade Last MAP kinase in cascade phosphorylates target proteins in the nucleus Activates them to turn on specific genes Many of these genes control cell division Mutations Mutated systems can turn on the pathways permanently, contributing to progression of some forms of cancer The importance of Ras Early 1980’s, several human tumors were found to contain a mutant of Ras Ras mutations are found in over 30% of all human tumors Mutation in the Ras gene that lead to tumor formation prevent the protein from hydrolzying the bound GTP back to GDP Ras always “on” The importance of Ras Ras is a small G protein held to the inner surface of the plasma membrane by a lipid group that is embedded in the inner leaflet of the bilayer Ras is a single subunit G protein Cycles between an active [GTP-bound] form and an inactive [GDP-bound] form Activates a kinase cascade (MAP Kinase) Gene Regulation Fig. 7-13, p. 151 Gene Activation: Steroid Hormone Receptors Fig. 7-14, p. 152 Cell Response Cell response to a steroid hormone Depends on whether it has an internal receptor for the hormone Type of response within the cell Depends on the genes that are recognized and turned on by an activated receptor Cross-Talk Cell signaling pathways communicate with one another to integrate responses to cellular signals May result in a complex network of interactions between cell communication pathways Cross-Talk Fig. 7-15, p. 153 Core signaling Pathways Many target proteins