* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download as a PDF
Complement system wikipedia , lookup
Gluten immunochemistry wikipedia , lookup
Osteochondritis dissecans wikipedia , lookup
Rheumatic fever wikipedia , lookup
DNA vaccination wikipedia , lookup
Drosophila melanogaster wikipedia , lookup
Atherosclerosis wikipedia , lookup
Behçet's disease wikipedia , lookup
Innate immune system wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Molecular mimicry wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Multiple sclerosis signs and symptoms wikipedia , lookup
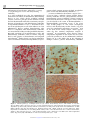
Multiple Sclerosis (1998) 4, 93 ± 98 1998 Stockton Press All rights reserved 1352 ± 4585/98 $12.00 http://www.stockton-press.co.uk/ms Neuropathology in multiple sclerosis: new concepts Hans Lassmann Institute of Neurology, University of Vienna, Austria Multiple sclerosis lesions are characterized by in¯ammation, demyelination and a variable degree of axonal loss. The patterns of in¯ammation in MS lesions are compatible with a T-lymphocyte mediated immune reaction. The formation of demyelinated plaques, however, seem to require additional immunological mechanisms. In this review evidence is discussed for a pathogenetic role of demyelinating antibodies, toxic macrophage products, cytotoxic T-cells as well as metabolic disturbances of oligodendrocytes. It is suggested that the pathological heterogeneity regarding the patterns and extent of demyelination, remyelination and axonal loss may be the outcome of variable dominant immunopathogenetic mechanisms in different multiple sclerosis patients. Keywords: Multiple sclerosis; pathology; demyelination; axon; oligodendrocyte Introduction Multiple sclerosis is a chronic in¯ammatory disease of the central nervous system, which is associated with selective destruction of myelin sheaths. This leads to the formation of large demyelinated plaques, dispersed throughout the entire brain and spinal cord. Axons in the plaques are relatively spared, although emphasis has to be laid on the term relative.1 In particular, in cases with progressive disease course and severe neurological de®cit axonal destruction and loss can be profound, in some cases affecting more than 80% of the axonal population in the plaques.2 Furthermore, the degree of axonal loss and atrophy more closely correlates with permanent clinical de®cit compared to the total amount of lesions or the extent of demyelination.3 The demyelinating process is counteracted by remyelination, at least in early stages of the disease.4,5 In such cases complete lesions may become remyelinated and may appear as so called `Markschattenherde'.4,6 The clinical spectrum of multiple sclerosis is broad, including relapsing-remittent, primary and secondary progressive and acute-subacute disease courses. Even more pronounced is the pathologic heterogeneity of the disease, in particular, when atypical disease variants, such as acute MS, neuromyelitis optica, concentric sclerosis and diffuse sclerosis are included in the spectrum.7 ± 9 Even the classical variants of chronic relapsing or progressive multiple sclerosis are neuropathologically re¯ected in a broad spectrum of different lesion subtypes.10 Recent studies suggest that this heterogenous spectrum of multiple sclerosis pathology may be due to a multitude of susceptibility genes in affected patients and to a wide spectrum of different immunological mechanisms, that may be responsible for in¯ammation and demyelination of the disease.11 ± 13 Correspondence: H Lassmann T-cell mediated in¯ammation is a characteristic feature of multiple sclerosis lesions, but alone is unlikely to cause the disease The in¯ammatory in®ltates in multiple sclerosis lesions have been characterized during the last years in detail. They are composed by T-lymphocytes, by small numbers of B-lymphocytes and plasma cells and ± in particular in actively demyelinating lesions ± by activated microglia or macrophages.14 ± 18 The latter cell population interacts with myelin sheaths and apparently is actively engaged in the demyelinating process. A variety of immune related molecules, such as histocompatibility antigens, adhesion molecules and cytokines are locally produced or expressed in the lesions.14,19 ± 22 These patterns suggest that a T-cell mediated immune response is the basis of brain in¯ammation in this disease. In experimental models a pure encephalitogenic T-cell mediated immune reaction in general leads to brain in¯ammation without demyelination.23,24 Similarly in humans, the extensive T-cell mediated in¯ammation, that is the hallmark of acute disseminated leucoencephalomyelitis, does not result in MS-like demyelinated plaques in the nervous system. For these reasons it is likely that additional, speci®c demyelinating ampli®cation factors are required to induce the full blown multiple sclerosis lesion. Mechanisms of demyelination Basic research in immunology and neurobiology revealed that a selective destruction of myelin sheaths can be induced by multiple different mechanisms. They include speci®c cellular and humoral immune mechanisms, the unspeci®c action of certain macrophage toxins as well as causes that reside in the metabolism of the myelin producing cells, the oligodendrocytes. All these mechanisms are potential candidates in the pathogenesis of multiple sclerosis. Downloaded from msj.sagepub.com at PENNSYLVANIA STATE UNIV on May 11, 2016 Neuropathology in multiple sclerosis: new concepts H Lassmann et al 94 In¯ammatory demyelination, induced by a synergy of encephalitogenic T-cells and demyelinating antibodies It is well established now that this immunological mechanism can induce an in¯ammatory demyelinating disease in vivo, which closely resembles multiple sclerosis. The basic principle of this immune reaction has been documented in detail by transferring encephalitogenic T-lymphocytes and demyelinating antibodies in experimental models in vivo.25,26 Intravenous injection of encephalitogenic T-cells alone induces an acute in¯ammatory disease without demyelination. This disease is ampli®ed, when demyelinating antibodies are cotransferred, leading to widespread demyelination. Repeated cotransfers of encephalitogenic T-cells and demyelinating antibodies result in chronic relapsing disease with plaques of demyelination and impaired remyelination.27 Similar lesions can also be induced by active sensitization with myelin oligodendrocyte glyco- protein, which contains epitopes for both, encephalitogenic T-cells and demyelinating antibodies.28 ± 30 A variety of different observations suggests that ± at least in some ± multiple sclerosis patients, demyelinating antibodies may be pathogenetically relevant. Demyelinating activity of multiple sclerosis serum has been found by Bornstein and Appel, which could in part be related to the action of immunoglobulins.31,32 Immunoglobulin precipitation occurs at the active edge of MS plaques, at sites where macrophages interact with disintegrating myelin sheaths.33 In these areas also complement components are present,34 even in the form of the C9neo antigen, which appears, when the lytic terminal complement complex is activated.74 In exceptionally severe disease courses, complement deposition in the lesions may be accompanied by granulocytes in the in¯ammatory in®ltrates (Figure 1c). It is thus likely, that in this subgroup of multiple sclerosis patients demyelinating antibodies Figure 1 Immunopathology of Multiple Sclerosis. (a) actively demyelinating lesions. PLP mRNA in oligodendrocytes (black cells) is absent in the area of active myelin destruction, characterized by macrophages with myelin degradation products (red granules); immunocytochemistry for PLP-protein (red) and in situ hybridization for PLP mRNA (black).6400. (b) Oligodendrocytes (immunostained with a-MOG antibody; brown) undergoing DNAfragmentation visualized by in situ tailing (black nuclei). Some of the labeled nuclei show condensed chromatin, similar to that found in apoptosis. 6900. (c) Exceptionally severe, destructive acute MS lesion with Complement C9neo deposition (red) and in®ltration of the tissue with granulocytes. 6600 Downloaded from msj.sagepub.com at PENNSYLVANIA STATE UNIV on May 11, 2016 Neuropathology in multiple sclerosis: new concepts H Lassmann et al are a pathogenetic ampli®cation factor, acting on the background of a T-cell mediated in¯ammatory response. The identi®cation of a combined T-cell and antibody mediated pathogenesis in some MS lesions has profound therapeutic consequences. Immunomodulatory strategies, aimed to suppress a Th1 mediated autoimmunity by stimulating the Th2 pathway may in this particular situation be counterproductive by increasing the pathogenic demyelinating antibody response.35 As an example, oral tolerization with myelin in primate chronic EAE may result in severe relapses after cessation of therapy.36 T-cell mediated destruction of myelin and oligodendrocytes Several in vitro studies suggest that myelin and oligodendrocytes can also be destroyed by T-cell mediated immune mechanisms. Adding activated Tcells to myelinated tissue culture systems can result in selective destruction of myelin and oligodendrocytes.37,38 This may be accomplished by T-cell products, such as lymphotoxin or perforin.39,40 In addition the activation of the Fas-signaling pathway in oligodendrocytes by its speci®c ligand on T-cells may destroy oligodendrocytes selectively.41 It is not clear as yet whether speci®c antigen recognition on the surface of oligodendrocytes ± in particular recognition of stress proteins ± may contribute to demyelination and destruction of myelin-forming cells.42,43 So far little evidence is available for a pathogenetic role of T-cell mediated demyelinating mechanisms in experimental models in vivo. In particular no model as yet has demonstrated that demyelinated plaques can be induced in vivo as a direct consequence of transfer of any T-cell population. In contrast, in multiple sclerosis lesions some indirect evidence accumulated, which suggests that such immune reactions indeed may be pathogenic. Fas receptor is expressed on a variety of different glia cells in multiple sclerosis lesions, in particular also on oligodendrocytes.41,44 The respective Fas-ligand is found on activated T-cells, but also on microglia cells.41 In addition, Fas-positive oligodendrocytes have been found ingested in macrophages or microglia.44 Another indirect evidence for a possible involvement of T-cell mediated immune reactions in demyelination is the observed colocalization of stress protein reactive oligodendrocytes with Tlymphocytes carrying gamma/delta T-cell receptors. From these data it was suggested that such T-cells may lyse stressed ± possibly remyelinating ± oligodendrocytes in established lesions.42 Macrophage mediated demyelination In vitro oligodendrocytes are particularly vulnerable to the action of macrophage toxins. Thus they can be selectively destroyed by complement components, reactive oxygen intermediates or cytokines, such as tumor necrosis factor alpha.45 ± 47 It was thus suggested that demyelination in multiple sclerosis may be a consequence of macrophage activation in the course of T-cell mediated brain in¯ammation. However, it has to be kept in mind that acute T-cell mediated in¯amma- tory CNS diseases, such as acute disseminated encephalomyelitis or acute virus induced encephalomyelitis, which in general are associated with profound in®ltration of the tissue by activated macrophages, do not lead to the formation of con¯uent demyelinated plaques. Therefore, macrophage mediated demyelination in multiple sclerosis is only feasible, when there is a unique dysregulation of macrophage toxicity or oligodendrocyte susceptibility. Transgenic mice, which overexpress tumor necrosis factor alpha in the central nervous system can ± under some circumstances ± develop plaques of in¯ammatory demyelination, which in many respects re¯ect multiple sclerosis lesions.48 In these lesions an early stage of vacuolar myelinopathy and oligodendroglia apoptosis is followed by widespread and plaque like destruction of myelin sheaths. Massive activation of macrophages is a cardinal feature of the pathology of active multiple sclerosis.18 In addition, the local expression of toxic macrophage products, such as tumor necrosis factor alpha has convincingly been documented in MS plaques.49,50 Recently we found also that in some cases of multiple sclerosis actively demyelinated lesions are not associated with deposition of immunoglobulin and complement, but are rather characterized by massive macrophage and microglia activation together with oligodendrocyte death by apoptosis (Figure 1b). Demyelination by metabolic instability of oligodendrocytes Metabolic instability of oligodendrocytes may either be induced by virus infection or by defects in the regulation of myelin genes. There is ample evidence that virus infection of oligodendrocytes directly may lead to cell death.51 In addition however, acute virus infection may disturb the expression of myelin gene expression. As an example, infection of myelinforming cells with both Theiler's virus and corona virus block the luxury function of these cells, the production of myelin proteins, such as proteolipid protein.52 ± 54 A different mechanism of metabolic instability of oligodendrocytes is exempli®ed in transgenic animals, which overexpress major myelin proteins, such as proteolipid protein during myelin formation.55,56 In this model, massive overexpression leads to oligodendrocyte death by apoptosis during the phase of myelination. However, a moderate overexpression can be balanced, resulting in only slow degeneration of oligodendrocytes, minor demyelination and repair of myelin. As another example, de®ciency of myelin associated glycoprotein does not prevent myelin formation but ± with increasing age of the animals ± is associated with a peculiar type of distal oligodendroglia damage, named `dying back' oligodendrogliopathy.57,58 It is possible, but so far not proven that such metabolic disturbances may render oligodendrocytes more susceptible to immune mediated injury. Evidence for an association between multiple sclerosis susceptibility and myelin gene polymorphism so far is controversial. Linkage of disease susceptibility with myelin basic protein gene polymorphism has Downloaded from msj.sagepub.com at PENNSYLVANIA STATE UNIV on May 11, 2016 95 Neuropathology in multiple sclerosis: new concepts H Lassmann et al 96 been disclosed, but did not become apparent in larger genomic screens. It may thus be restricted to a special cohort of multiple sclerosis patients. Similarly, virus infection of oligodendrocytes has sometimes been observed in multiple sclerosis lesions although no particular MS-speci®c virus infection has been found so far.59 Downregulation of the expression of myelin protein mRNAs is frequent in active MS plaques (Figure 1a) and on the protein basis in some cases a pronounced loss of myelin associated glycoprotein can be found.60 The alterations of `dying back' oligodendrogliopathy have been identi®ed in virus induced experimental demyelinating diseases as well as in some multiple sclerosis lesions.61 Finally, in some patients with chronic progressive multiple sclerosis we found a pattern of demyelination, suggesting a primary oligodendrocyte destruction, followed by demyelination.62,70 All these data suggest that metabolic instability of oligodendroglia may occur in certain MS lesions, its consequence for the pathogenesis of demyelination, however, is as yet unde®ned. Mechanisms of axonal damage As mentioned above axons are destroyed to a variable degree in multiple sclerosis plaques. This is a major problem in MS pathogenesis, since axonal loss signi®cantly contributes to permanent functional de®cit. In contrast to demyelination little attention in MS research has been paid to the problem of axonal pathology. From pathological studies it is evident, that the extent and severity of the in¯ammatory reaction is to some extent related to the amount of axonal damage.63,64 As an example, the most extensive loss of axons is found in plaques of Marburg's acute MS, which are also characterized by a much more severe nature of the in¯ammatory process, compared to classical chronic MS. In experimental models axonal damage can be induced by products of activated macrophages. These cells are a major source of endogenous excitotoxins, which may be responsible for neuronal injury.65 Excitotoxins however exert their damaging effect through glutamate receptors, which are rather distributed on dendrites and neuronal pericarya than on the axons themselves. Thus excitotoxicity rather results in axon sparing neuronal damage than to direct axonal pathology.66 Functional damage of axons can also be induced by nitric oxide and this effect is more pronounced in demyelinated compared to intact nerve ®bers.67 It is, however, not clear so far whether nitric oxide can also be responsible for structural and permanent axonal destruction. In MS pathology acute axonal damage in general takes place during the period of lesional activity, although it seems to follow the destruction of myelin sheaths. The reason for the profound differences in the extent of axonal loss between different multiple sclerosis patients, however, remains to be elucidated. Remyelination and lesional repairs The occurrence of spontaneous remyelination in multiple sclerosis lesions has been suggested already in the earliest pathological descriptions. As an example Marburg drew the attention to the occurrence of very thin myelin sheaths in acute MS lesions, which were only detectable after osmic acid impregnation of the sections.1 Although at that time no experimental evidence for remyelination in the central nervous system was available, Marburg suggested a remyelinating process in analogy to the patterns of peripheral nerve regeneration. It is now well established that extensive remyelination may occur in multiple sclerosis spontaneously, forming the so called `shadow plaques, which have primarily been de®ned by Schlesinger as incompletely demyelinated areas. Remyelination is a particular feature of early MS lesions, which arise during the ®rst years after onset of the disease and are abundantly found in biopsies taken at this stage.4 ± 6,10,62,68 ± 70 The extent of remyelination in MS lesions apparently is determined by two factors: the availability of oligodendrocytes or progenitor cells and the degree of axonal damage in the lesions. Although oligodendrocytes may be preserved during the acute stage of demyelination in MS lesions it is doubted that these cells are responsible for remyelination.62,70 Recent experimental studies rather suggest that the pool of remyelinating cells is recruited from undifferentiated glial progenitor cells.71 Lack of remyelination in chronic MS plaques may thus be explained by repeated de- and remyelination in the same lesion and exhaustion of the progenitor cell fraction.72 Besides this, however, the plaque environment ± such as the degree of astroglial scar formation or the lack of proper trophic support ± may also impede remyelination. In line with this notion, chronic MS plaques not infrequently contain signi®cant numbers of poorly differentiated oligodendrocytes in the absence of remyelination. Such cells potentially could be activated to start lesional repair by therapeutic intervention.73 Heterogeneity of multiple sclerosis pathology suggests the involvement of different pathogenetic mechanisms As discussed above many different pathogenetic mechanisms can be involved in demyelination, remyelination and axonal damage in multiple sclerosis lesions. It is thus not surprising that active multiple sclerosis lesions reveal a broad heterogeneity in their neuropathological characteristics. With regard to structural aspects of demyelination, oligodendrocyte loss, remyelination and axonal pathology we have recently classi®ed multiple sclerosis lesions into at least ®ve different subtypes: (a) demyelination with no or only minor oligodendrocyte loss; (b) demyelination with concomitant destruction and loss of oligodendrocytes; (c) primary demyelination with a secondary gradient loss of oligodendrocytes in inactive plaque areas; (d) primary oligodendrocyte destruction in the periplaque white matter and secondary demyelination and (e) destructive lesions with profound additional loss of axons and in part of astrocytes.10 Since all the lesions were classi®ed with the same stringent criteria of Downloaded from msj.sagepub.com at PENNSYLVANIA STATE UNIV on May 11, 2016 Neuropathology in multiple sclerosis: new concepts H Lassmann et al lesional activity it is unlikely that these different pathological patterns represent speci®c stages in the evolution of the lesions. Similarly, the severity of the immunological reaction was unlikely to be an explanation for this structural heterogeneity. Ongoing immunopathological studies rather suggest that demyelination may be induced by different immunological mechanisms and that this will also result in distinct patterns of structural pathology. Furthermore the lesional pro®le was highly conserved in different plaques of a single patient, while major differences were present in the pathological patterns between different patients. This indicates, that different multiple sclerosis patients may preferentially use different immunopathogenetic pathways of demyelination in their lesions. It will be a major task in future MS research to ®nd clinical and paraclinical markers that allow to differentiate such pathogenetic subtypes of the disease and design therapeutic strategies, which speci®cally target the diverse mechanisms responsible for in¯ammation, demyelination and axonal destruction. Acknowledgements This study was funded by a project of the Austrian Science Foundation. References 1 Marburg O. (1906) Die sogenannte `akute Multiple Sklerose'. Jahrb Psychiatrie 27: 211 ± 312. 2 Lassmann H. (1997) Multiple sclerosis pathology. In: A. Compston (Ed) McAlpine's Multiple Sclerosis, 3rd Edition; Churchill Livingstone in press. 3 Losseff NA et al. (1996) Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 119: 701 ± 708. 4 Lassmann H. (1983) Comparative neuropathology of chronic experimental allergic encephalomyelitis and multiple sclerosis. Schriftenr Neurol 25: 1 ± 35. 5 Prineas JW et al. (1993a) Multiple sclerosis: remyelination of nascent lesions. Ann Neurol 33: 137 ± 151. 6 Schlesinger H. (1909) Zur Frage der akuten multiplen Sklerose und der encephalomyelitis disseminata im Kindesalter. Arb Neurol Inst (Wien) 17: 410 ± 432. 7 De vic C. (1994) Mye lite subaigue complique e de ne vrite optique. Bull Med 8: 1033. 8 Balo J. (1928) Encephalitis periaxialis concentrica. Arch Neurol 19: 242 ± 264. 9 Schilder P. (1912) Zur Kenntnis der sogenannten diffusen Sklerose (uÈ ber Encephalitis periaxialis diffusa). Z Gesamt Neurol Psychiatr 10: 1 ± 60. 10 Lucchinetti CF, BruÈ ck W, Rodriguez M, Lassmann H. (1996) Distinct patterns of multiple sclerosis pathology indicates heterogeneity in pathogenesis. Brain Pathol 6: 259 ± 274. 11 Haines JL et al. (1996) A genomic screen for multiple sclerosis underscores a role for the major histocompatibility complex. The multiple sclerosis genetics group. Nature Genetics 13: 469 ± 471. 12 Sawcer S et al. (1996) A genomic screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nature Genetics 13: 464 ± 468. 13 Ebers GC et al. (1996) A full genome search in multiple sclerosis. Nature Genetics 13: 472 ± 476. 14 Traugott U, Reinherz EL, Raine CS. (1983) Multiple sclerosis. Distribution of T cells, T cell subsets and Iapositive macrophages in lesions of different ages. J Neuroimmunol 4: 201 ± 221. 15 Esiri MM. (1977) Immunoglobulin-containing cells in multiple sclerosis plaques. Lancet 2: 478 ± 480. 16 Newcombe J, Li H, Cuzner ML. (1994) Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: implications for pathogenesis. Neuropathol Apll Neurobiol 20: 152 ± 162. 17 Ulvestad E et al. (1994) Reactive microglia in multiple sclerosis lesions have an increased expression of receptors for the Fc part of IgG. J Neurol Sci 121: 125 ± 131. 18 BruÈ ck W et al. (1995) Monocyte/macrophage differentiation in early multiple sclerosis. Ann Neurol 38: 788 ± 796. 19 Washington R et al. (1994) Expression of immunologically relevant endothelial cell activation antigens on isolated central nervous system microvessels from patients with multiple sclerosis. Ann Neurol 35: 89 ± 97. 20 Cannella B, Raine CS. (1995) The adhesion molecule and cytokine pro®le of multiple sclerosis lesions. Ann Neurol 37: 424 ± 435. 21 Wucherpfenning KW et al. T-cell receptor V alpha-V beta repertoire and cytokine gene expression in active multiple sclerosis lesions. J Exp Med 175: 993 ± 1002. 22 Woodroofe MN, Cuzner ML. (1993) Cytokine mRNA expression in in¯ammatory multiple sclerosis lesions: detection by nonradioactive in situ hybridization. Cytokine 5: 583 ± 588. 23 Ben-Nun A, Wekerle H, Cohen IR. (1981) The rapid isolation of clonable antigen-speci®c T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol 11: 195 ± 199. 24 Berger T et al. (1997) Experimental autoimmune encephalomyelitis: The antigen speci®city of T-lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Invest 76: 97 ± 108. 25 Linington C, Bradl M, Lassmann H, Brunner C, Vass K. (1988) Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol 130: 443 ± 454. 26 Lassman H, Brunner C, Bradl M, Linington C. (1988) Experimental allergic encephalomyelitis: The balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol 75: 566 ± 576. 27 Linington C, Engelhardt B, Kapocs G, Lassmann H. (1992) Induction of persistently demyelinating lesions in the rat following the repeated adoptive transfer of encephalitogenic T cells and demyelinating antibodies. J Neuroimmunol 40: 219 ± 224. 28 Amor S et al. (1994) Identi®cation of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol 153: 4349 ± 4356. 29 Adelmann M et al. (1995) The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J Neuroimmunol 63: 17 ± 27. 30 Johns TG et al. (1995) Myelin oligodendrocyte glycoprotein induces a demyelinating encephalomyelitis resembling multiple sclerosis. J Immunol 154: 5536 ± 5541. 31 Bornstein MB, Appel SH. (1965) Tissue culture studies of demyelination. Ann NY Acad Sci 122: 280 ± 286. 32 Grundke Iqbal I, Bornstein MB. (1980) Multiple sclerosis: serum gamma globulin and demyelination in culture. Neurology 30: 749 ± 754. Downloaded from msj.sagepub.com at PENNSYLVANIA STATE UNIV on May 11, 2016 97 Neuropathology in multiple sclerosis: new concepts H Lassmann et al 98 33 Prineas JW, Graham JS (1981) Multiple sclerosis: capping of surface immunoglobulin G on macrophages engaged in myelin breakdown. Ann Neurol 10: 149 ± 158. 34 Gay D, Esiri M. (1991) Blood-brain barrier damage in acute multiple sclerosis plaques. An immunocytochemical study. Brain 114: 557 ± 572. 35 Ha¯er DA, Weiner HL. (1995) Immunologic mechanisms and therapy in multiple sclerosis. Immunol Rev 144: 75 ± 107. 36 Genain CP et al. (1996) Late complications of immune deviation therapy in a nonhuman primate. Science 274: 2054 ± 2057. 37 D'Souza S, Alinauskas K, McCrea E, Goodyer C, Antel JP. (1995) Differential susceptibility of human CNS-derived cell populations to TNF dependent and independent immune-mediated injury. J Neurosci 15: 7293 ± 7300. 38 Freedman MS, Ruijs TC, Selin LK, Antel JP. (1991) Peripheral blood gamma/delta T cells lyse fresh human brain derived oligodendrocytes. Ann Neurol 30: 794 ± 800. 39 Selmaj K et al. (1991b) Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J Immunol 147: 1522 ± 1529. 40 Scolding N, Jones J, Compston DA, Morgan BP. (1990) Oligodendrocyte susceptibility to injury by T-cell perforin. Immunology 70: 6 ± 10. 41 D'Souza SD et al. (1996) Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med 184: 2361 ± 2370. 42 Selmaj K, Brosnan CF, Raine CS. (1991a) Colocalization of lymphocytes bearing yd T-cell receptor and heat shock protein hsp65+ oligodendrocytes in multiple sclerosis. Proc Natl Acad Sci USA 88: 6452 ± 6456. 43 van Noort JM et al. (1995) A novel candidate autoantigen in multiple sclerosis: aB-crystallin, a small heat shock protein. Nature 375: 798 ± 801. 44 Dowling P et al. (1996) Involvement of the CD95 (APO-1/ Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med 184: 1513 ± 1518. 45 Scolding NJ et al. (1989). Normal rat serum cytotoxicity against syngeneic oligodendrocytes: Complement activation and attack in the absence of anti-myelin antibodies. J Neurol Sci 89: 289 ± 300. 46 Griot C et al. (1990) Selective degeneration of oligodendrocytes mediated by reactive oxygen species. Free Radic Res Commun 11: 181 ± 193. 47 Selmaj KW, Raine CS. (1988) Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol 23: 339 ± 346. 48 Probert L et al. (1995) Spontaneous in¯ammatory demyelinating disease in transgenic mice showing central nervous system-speci®c expression of tumor necrosis factor a. Proc Natl Acad Sci USA 92: 11294 ± 11298. 49 Hofman FM, Hinton DR, Johnson K, Merrill JE. (1989) Tumor necrosis factor identi®ed in multiple sclerosis brain. J Exp Med 170: 607 ± 612. 50 Selmaj K, Raine CS, Cannella B, Brosnan CF. (1991c) Identi®cation of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest 87: 949 ± 954. 51 Rosenthal A, Fujinami RS, Lampert PW. (1986) Mechanisms of Theiler's virus induced demyelination in nude mice. Lab Invest 54: 515 ± 522. 52 Jordan CA et al. (1989) Expression of viral and myelin gene transcripts in a murine CNS demyelinating disease caused by a coronavirus. Glia 2: 318 ± 329. 53 Rodriguez M, Prayoonwiwat N, Howe C, Sanborn K. (1994) Proteolipid protein gene expression in demyelination and remyelination of the central nervous system: a model for multiple sclerosis. J Neuropath Exp Neurol 53: 136 ± 143. 54 Barac-Latas et al. (1997) Patterns of oligodendroglia pathology in corona virus induced subacute demyelinating encephalomyelitis in the Lewis rat. Glia 19: 1 ± 12. 55 Readhead C, Schneider A, Grif®ths I, Nave KA. (1994) Premature arrest of myelination in transgenic mice with increased proteolipid protein gene dosage. Neuron 12: 583 ± 595. 56 Kagawa T et al. (1994) Glial cell degeneration and hypomyelination caused by overexpression of the myelin proteolipid gene. Neuron 13: 427 ± 442. 57 Montag D et al. (1994) Mice de®cient for the myelinassociated glycoprotein show subtle abnormalities in myelin. Neuron 13: 229 ± 246. 58 Lassmann H, Bartsch U, Montag D, Schachner M. (1997) Dying back oligodendrogliopathy: A late sequal of myelin-associated glycoprotein de®ciency. Glia 19: 104 ± 110. 59 Challoner PB et al. (1995) Plaque associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA 92: 7440 ± 7444. 60 Gendelman HE et al. (1985) A quantitation of myelinassociated glycoprotein and myelin basic protein loss in different demyelinating diseases. Ann Neurol 18: 324 ± 328. 61 Rodriguez M, Scheithauer BW, Forbes G, Kelly PJ (1993) Oligodendrocyte injury is an early event in lesions of multiple sclerosis. Mayo Clin Proc 68: 627 ± 636. 62 Ozawa K et al. (1994) Patterns of oligodendroglia pathology in multiple sclerosis. Brain 117: 1311 ± 1322. 63 Lassmann H, Suchanek G, Ozawa K. (1994) Histopathology and the blood-cerebrospinal ¯uid barrier in multiple sclerosis. Ann Neurol 36: S42 ± S46. 64 BruÈ ck W et al. (1997) In¯ammatory central nervous system demyelination: correlation of magnetic resonance imaging ®ndings with lesion pathology. Ann Neurol in press. 65 Heyes MP, Saito K, Markey SP. (1992) Human macrophages convert L-tryptophan into the neurotoxin quinolinic acid. Biochem J 283: 633 ± 635. 66 Olney JW. (1983) Excitotoxins: an overview. In: K Fuxe, P Roberts and R Schwarcz (Eds). Excitotoxins Macmillan: London. 82 ± 96. 67 Chou SM, Wang HS, Taniguchi A. (1996) Role of SOD-1 and nitric oxide/cyclic GMP cascade on neuro®lament aggregation in ALS/MND. J Neurol Sci 139: 16 ± 26. 68 Raine CS, Scheinberg L, Waltz JM. (1981) Multiple sclerosis: Oligodendrocyte survival and proliferation in an active established lesion. Lab Invest 45: 534 ± 546. 69 Prineas JW, Kwon EE, Cho ES, Sharer LR. (1984) Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann NY Acad Sci 436: 11 ± 32. 70 BruÈ ck W et al. (1994) Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol 35: 65 ± 73. 71 Targett MP et al. 1996) Failure to achieve remyelination of demyelinated rat axons following transplantation of gloal cells obtained from the adult human brain. Neuropathol Appl Neurobiol 22: 199 ± 206. 72 Prineas JW et al. (1993b) Multiple sclerosis. Pathology of recurrent lesions. Brain 116: 681 ± 693. 73 Miller DJ, Asakura K, Rodriguez M. (1996). Central nervous system remyelination. Clinical application of basic neuroscience principles. Brain Pathol 6: 331 ± 344. 74 Storch MK et al. (1998) Multiple sclerosis: In situ evidence for antibody ± and complement mediated demyelination. Ann Neurol 43: 465 ± 471. Downloaded from msj.sagepub.com at PENNSYLVANIA STATE UNIV on May 11, 2016