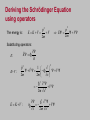* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 6. Quantum Mechanics II
Bohr–Einstein debates wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Probability amplitude wikipedia , lookup
Coherent states wikipedia , lookup
Path integral formulation wikipedia , lookup
Renormalization group wikipedia , lookup
Perturbation theory (quantum mechanics) wikipedia , lookup
Density matrix wikipedia , lookup
Compact operator on Hilbert space wikipedia , lookup
Tight binding wikipedia , lookup
Self-adjoint operator wikipedia , lookup
Coupled cluster wikipedia , lookup
Canonical quantization wikipedia , lookup
Hydrogen atom wikipedia , lookup
Erwin Schrödinger wikipedia , lookup
Dirac equation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Wave–particle duality wikipedia , lookup
Matter wave wikipedia , lookup
Particle in a box wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Schrödinger equation wikipedia , lookup
Wave function wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
CHAPTER 5 The Schrodinger Eqn. 5.1 5.2 5.3 5.4 5.5 The Schrödinger Wave Equation Expectation Values Infinite Square-Well Potential Finite Square-Well Potential Three-Dimensional InfinitePotential Well 5.6 Simple Harmonic Oscillator 5.7 Barriers and Tunneling Erwin Schrödinger (1887-1961) Homework due next Wednesday Oct. 1st Read Chapters 4 and 5 of Kane Chapter 4: 2, 3, 5, 13, 20 Chapter 5: 3, 4, 5, 7, 8 Stationary States The wave function can now be written as: ( x, t ) ( x)eiEt / ( x)eit The probability density becomes: * * ( x) eit ( x) eit ( x) 2 The probability distribution is constant in time. This is a standing-wave phenomenon and is called a stationary state. Most important quantum-mechanical problems will have stationary-state solutions. Always look for them first. Operators d 2 V E 2 2m dx 2 The time-independent Schrödinger wave equation is as fundamental an equation in quantum mechanics as the timedependent Schrödinger equation. So physicists often write simply: Ĥ E where: 2 Hˆ V 2 2m x 2 Ĥ is an operator yielding the total energy (kinetic plus potential energies). Operators 2 d 2 ( x) V ( x) ( x) E ( x) 2 2m dx Operators are important in quantum mechanics. All observables (e.g., energy, momentum, etc.) have corresponding operators. The kinetic energy operator is: 2 K 2m x 2 2 Other operators are simpler, and some just involve multiplication. The potential energy operator is just multiplication by V(x). Momentum Operator To find the operator for p, consider the derivative of the wave function of a free particle with respect to x: i ( kx t ) [e ] ikei ( kx t ) ik x x p With k = p / ħ we have: i x This yields: p i x This suggests we define the momentum operator as: pˆ i The expectation value of the momentum is: p i * ( x, t ) ( x, t ) dx x . x Position and Energy Operators The position x is its own operator. Done. Energy operator: Note that the time derivative of the free-particle wave function is: i ( kx t ) [e ] iei ( kx t ) i t t Substituting E / ħ yields: E i t This suggests defining the energy operator as: Eˆ i t The expectation value of the energy is: E i ( x, t ) ( x, t ) dx t * Deriving the Schrödinger Equation using operators The energy is: p2 E V 2m p2 E K V V 2m Substituting operators: Ê i E: t pˆ 2 1 Vˆ i V 2m 2m x 2 K+V : 2 V 2 2m x 2 E K V : 2 2 i V 2 t 2m x Operators and Measured Values In any measurement of the observable associated with an operator A, ˆ the only values that can ever be observed are the eigenvalues. Eigenvalues are the possible values of a in the Eigenvalue Equation: Â a where a is a constant and the value that is measured. For operators that involve only multiplication, like position and potential energy, all values are possible. But for others, like energy and momentum, which involve operators like differentiation, only certain values can be the results of measurements. In this case, the function is often a sum of the various wave function solutions of Schrödinger’s Equation, which is in fact the eigenvalue equation for the energy operator. Solving the Schrödinger Equation when V is constant. When V0 > E: d 2 V0 E 2 2m dx 2 d 2 2m Rearranging: 2 V0 E 2 dx d 2 2 a 2 dx where: a 2m V0 E / 2 Because the sign of the constant a2 is positive, the solution is: kx kx 1 cosh( kx ) ( e e ) 2 ax a x Sometimes ( x) Ae Be When E > V0: d 2 2 k 2 dx people use: sinh( kx) 1 (e kx e kx ) 2 where: k 2m( E V0 ) / 2 Because the sign of the constant k2 is negative, the solution is: ( x) Aeikx Beikx or A sin(kx) B cos(kx) Infinite Square-Well Potential Consider a particle trapped in a box with infinitely hard walls that the particle cannot penetrate. This potential is called an infinite square well and is given by: V ( x) 0 x 0, x L 0 x L 0 L x Outside the box, where the potential is infinite, the wave function must be zero. Inside the box, where the potential is zero, the energy is entirely kinetic, E>V0 So, inside the box, the solution is: ( x) A sin(kx) B cos(kx) Taking A and B to be real. where k 2mE / 2 Quantization and Normalization Boundary conditions dictate that the wave function must be zero at x = 0 and x = L. This yields solutions for integer values of n such that kL = np. The wave functions ( x) A sin np x n are: L 0 L x The same functions as those for a vibrating string with fixed ends! In QM, we must normalize the wave functions: ( x) n ( x) dx 1 A * n The normalized wave functions become: 2 L 0 ½ ½ cos(2npx/L) np x sin dx 1 L n ( x) 2 2 npx sin L L A 2/ L Quantized Energy We say that k is quantized: Solving for the energy yields: kn 2mEn np L 2 En n 2 p2 2 2 2mL (n 1, 2, 3,...) The energy also depends on n. So the energy is also quantized. The special case of n = 1 is called the ground state. E1 p 2 2 2mL2 Finite Square-Well Potential The finite square-well potential is: V0 V ( x) 0 V 0 x 0 Region I 0 x L Region II x L Region III Assume: E < V0 The solution outside the finite well in regions I and III, where E < V0, is: Region I, x 0 I ( x) Aea x III ( x) Bea x Region III, x L Realizing that the wave function must be zero at x = ±∞.