* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Review: theory vs law the atomic theory contributions of early scientists
Survey
Document related concepts
Einsteinium wikipedia , lookup
Livermorium wikipedia , lookup
History of molecular theory wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Periodic table wikipedia , lookup
Valley of stability wikipedia , lookup
Chemical element wikipedia , lookup
Transcript
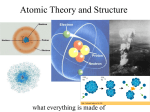
Review: theory vs law the atomic theory contributions of early scientists Oct 711:27 AM The Atom the smallest particle of an element into which it can be subdivided an atom consists of a nucleus which contains protons and neutrons, as well there are electrons which orbit the atom the nucleus is so tiny it would take 10,000 of them to stretch across the atom (the nucleus is like a grain of sand on a football field which represents the atom) the nucleus is always positive because of protons Oct 711:28 AM 1 Structure of an Atom Oct 711:33 AM Protons, Neutrons and Electons Particle Relative Charge Mass Location in Atom Heavy Positive (similar to neutrons) Inside the nucleus Proton Electron Light Negative Orbiting the nucleus Neutral Inside the Neutron Heavy (similar to nucleus protons) Oct 711:36 AM 2 Element a pure substance that cannot be broken down into smaller components all elements use symbols consisting of one or two letters (first letter is capitalized , second is not) all elements are listed on the Periodic Table of Elements (see page 50) this table was started by Dmitri Mendeleev who first sorted the elements by properties until he found a pattern (see page 48) Mendeleev also realized that there were some elements that may have existed but were not yet discovered. That is why the table will often have gaps to show elements that should be there Oct 71:42 PM Oct 71:53 PM 3 students will be required to know the chemical symbols for the following elements 1. Hydrogen 2. Sodium 3. Potassium 4. Magnesium 5. Calcium 6. Iron 7. Nickel 8. Copper 9. Zinc 10. Carbon 11. Nitrogen 12. Oxygen 13. Neon 14. Helium 15. Chlorine 16. Silicon 17. Silver 18. Gold 19. Mercury 20. Lead Oct 71:55 PM Oct 72:11 PM 4 Atomic Number equals the number of protons on a nucleus always a whole number, usually the top number in a Periodic Table Atomic Mass the average mass of the atoms of an element written as a decimal number, usually below the element symbol Oct 72:17 PM Example 14 Atomic Number Si Element Symbol Element Name Silicon Atomic Mass 28.1 Oct 72:19 PM 5 Determining protons, neutrons and electrons the protons of an element are the same as its atomic number the electrons equal the number of protons the neutrons are equal to atomic mass subtract atomic number (round down the atomic mass) Oct 72:24 PM Example: Determine the protons, neutrons and electrons in the following Element: Symbol: P E N Copper Sodium Oxygen Chlorine Oct 72:27 PM 6 The Element Song Readings: Pages 38 49 Questions: Page 47, #'s 1,2,6 Page 49, complete table in 22A Activities: Element Crossword and Wordsearch Oct 72:43 PM 7