* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Molecular characteristics of sucrose synthase
Artificial gene synthesis wikipedia , lookup
Protein adsorption wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Protein moonlighting wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Bottromycin wikipedia , lookup
Plant breeding wikipedia , lookup
Expression vector wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Gel electrophoresis wikipedia , lookup
Molecular evolution wikipedia , lookup
Protein–protein interaction wikipedia , lookup
List of types of proteins wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
History of molecular evolution wikipedia , lookup
Protein purification wikipedia , lookup
Biochemistry wikipedia , lookup
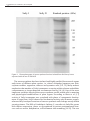
Molecular characteristics of sucrose synthase isolated from bird cherry leaves 41 Molecular characteristics of sucrose synthase isolated from bird cherry leaves Hubert Sytykiewicz*, Paweł Czerniewicz, Bogumił Leszczyński Department of Biochemistry and Molecular Biology University of Podlasie Prusa 12 08-110 Siedlce, Poland *corrresponding author: phone/fax: +4825 6431367, e-mail: [email protected] Summary The molecular characteristics of partly purified sucrose synthase (NDP-glucose: D-fructose 2-α-D-glucosyltransferase, EC 2.4.1.13), extracted from the bird cherry (Prunus padus L.) leaves was elucidated. The sucrose synthase was successfully purified by using a four-step protocol including ammonium sulfate precipitation, dialysis, gel filtration on Sephadex G-150 and ion-exchange chromatography with the use of DEAE-cellulose. The analysed enzyme occurred in two isoforms (SuSyI and SuSyII). The relative molecular weight of native isoenzymes was estimated to be 200 and 180 kDa, respectively. Isoform SuSyI contained two different subunits of 57.5 and 52.8 kDa, whereas the structure of SuSyII was consisted of identical 63 kDa subunits. Experimental data indicated that the structure of both SuSy isoforms was composed of three subunits. Key words: sucrose synthase, Prunus padus, molecular mass, subunit composition introduction The purpose of presented studies was to elucidate molecular characteristics of partly purified sucrose synthase extracted from the bird cherry leaves (Prunus padus L., Rosaceae). Sucrose is a major transport form of reduced carbon within the most higher plants [1, 2]. Sucrose synthase (SuSy, NDP-glucose: D-fructose 2-α-D-glucosyltransferase, EC 2.4.1.13) plays a substantial role in regulation of intracellular homoestasis and ontogenetic development of plants. Its subcellular localisation is not restricted to cytosolic or vacuol compartments, but also may occur as membrane-bound and associated to actin cytoskeleton forms [3-5]. Vol. 54 No 3 2008 H. Sytykiewicz, P. Czerniewicz, B. Leszczyński 42 Biochemical and physiological importance of the analysed enzyme is connected with its unique functions. The reaction catalysed by SuSy is completely reversible and depends on metabolic requirements of the cells or tissues. The equilibrium state in vivo may be displaced into direction of sucrose synthesis or degradation [6, 7]. The hypothesis that a broad spectrum of SuSy isoforms fulfill distinct metabolic functions is supported by the existence of multiple isoenzymes in many plant species that possess differentiated structure properties. On the other hand, level of expression and catalytic activity of those isoforms are influenced by tissue and subcellular localisation, phase of plant development and environmental factors (especially the presence of abiotic or biotic stressors) [7, 8]. MATERIAL AND METHODS Plant material Fresh leaves of the bird cherry grown in Aleksandria Park, Siedlce (central-eastern Poland) were used in the experiments. Tested organs were at sixth flushing stage (completely mature and fully expanded) in the Carter’s scale [9]. The plant material was placed in solid carbon dioxide, immediately transferred to laboratory and subjected to biochemical analyses. Enzyme isolation Extraction of the sucrose synthase from P. padus leaves was carried out with the use of Röhring et al. method [10]. Analysed organs were cut and homogenized at 4°C with Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2, 0.5 mM CaCl2, 1 mM EDTA, 250 mM sucrose, 5 mM DTT (dithiothreitol) and 1 mM PMSF (phenylmethylsulfonyl fluoride). Obtained homogenate was subsequently filtered through four layers of cheesecloth and centrifuged for 30 min. at 13,500 g. Pellets were discarded, and supernatants were used for further analyses. Sucrose synthase purification a) ammonium sulfate precipitation and dialysis Solid ammonium sulfate (35.4 g) was added to each portion of the supernatant (100 cm3) to obtain 30% saturation. Precipitated proteins were centrifuged for 30 min at 13,500 g. Supernatant was decanted and ammonium sulfate was added to obtain 55% saturation. The mixture was centrifuged as described above. Supernatant was discarded and the pellet was dissolved in extraction buffer at a temperature of 4°C. Subsequently, the solution was subjected to dialysis against Tris-HCl buffer (pH 7.5) for 6 h. Molecular characteristics of sucrose synthase isolated from bird cherry leaves 43 b) gel filtration Further phase of purification which consisted of separation of SuSy isoenzymes and determination of molecular mass of native isoforms was accomplished with use of Klotz et al. method [11]. Dialysates (each portion of 10 cm3) were loaded onto a glass column (2 × 40 cm) filled with Sephadex G-150 bed and equilibrated with 200 cm3 of extraction buffer. Enzyme elution was performed with the same buffer at the flow-rate of 0.8 cm3 · min-1. The eluates were collected in portions of 2 cm3 and the fractions of SuSy activity were pooled. Molecular weight of native SuSy isoforms (in kDa) was calculated with the use of experimentally outlined calibration curve showing relationship between Kav (coefficient of partitioning) and log10 molecular mass of the used standard proteins. c) ion-exchange chromatography The pooled gel filtration fractions were applied onto a glass column (1 x 34 cm) filled with DEAE-cellulose, pre-equilibrated with extraction buffer. Elution of SuSy isoenzymes were performed by means of a linear NaCl gradient (0-500 mM) at the flow-rate 0.3 cm3 · min-1. The eluates exhibiting SuSy activity were pooled in two separate fractions. Determination of SuSy subunit composition Evaluation of subunit composition of tested isoenzymes was determined electrophoretically with the use of SDS-PAGE technique. Separation of SuSy isoforms was carried out on 12.5% polyacrylamide gel by the procedure of Laemmli [12]. The standard discontinuous system under denaturing conditions (with the application of sodium dodecyl sulfate) was used. After electrophoresis, protein subunits were visualized within gel by staining with Coomassie Brilliant Blue R-250. Enzyme activity assay The specific activity of the analysed enzyme towards the cleavage direction was calculated as μg reducing sugars · min-1 · mg protein, whereas activity in the direction of sucrose synthesis was expressed as a loss in μg reducing sugars· min-1 · mg protein. Quantitative analyses of reducing sugars within the enzyme preparates were conduncted according to the standard method of SomogyiNelson [13]. The protein content was measured by using the method of Lowry et al. [14]. Vol. 54 No 3 2008 H. Sytykiewicz, P. Czerniewicz, B. Leszczyński 44 RESULTS AND DISCUSSION The performed purification techniques (ammonium sulfate precipitation, dialysis, gel filtration on Sephadex G-150 and ion-exchange chromatography with the use of DEAE-cellulose) allowed to obtain near-homogenous preparate of the sucrose synthase (tab. 1, fig. 1-2). Two different peaks that possessed SuSy activity were identified in the gel filtration (Sephadex G-150) step, and the two isoforms were named in order of elution as SuSyI and SuSyII (fig.1). After the ion-exchange chromatography on DEAE-cellulose, specific activity of the analysed SuSy isoforms slightly increased (approximately 2-fold increase of activity in relation to the prior phase of purification, see fig. 2). Figure 1. Fractions of SuSy obtained from bird cherry leaves, eluted from chromatographic column filled with Sephadex G-150 (A and B – fractions that possess activity of SuSy, subsequent peaks represent other biomolecules) Figure 2. Fractions of sucrose synthase isolated from P. padus leaves eluted from column filled with DEAE-cellulose (I, II –protein fractions that possess activity of SuSy, III, IV –other protein fractions) Molecular characteristics of sucrose synthase isolated from bird cherry leaves 45 Ta b l e 1 . Purification of sucrose synthase isolated from bird cherry leaves purification phase crude extract ammonium sulfate precipitation dialysis fraction A sephadex G-150 fraction B fraction I DEAE-cellulose fraction II protein content (mg ∙ cm-3) 120.00 98.20 40.02 12.40 10.30 6.20 5.80 specific activity of sucrose synthase cleavage synthesis reaction reaction 1.13 0.39 4.70 2.61 6.35 5.55 8.55 8.26 9.02 8.77 20.56 19.40 23.90 22.60 purification efficiency (n-fold) cleavage synthesis reaction reaction 1.00 1.00 4.16 6.69 5.62 14.23 7.58 21.17 8.00 22.49 18.20 49.74 21.19 57.95 The relative molecular mass of the native sucrose synthase isoenzymes was estimated to be 200 kDa (Kav=0.12) and 180 kDa (Kav=0.14), respectively (fig. 3). Electrophoretic separation of identified SuSy isoenzymes was carried out in discontinuous system under denaturing conditions, using the SDS-PAGE technique. Analyses of the electropherograms revealed that isoform SuSyI contained two different subunits of 57.5 kDa (Rf=0.36) and 52.8 kDa (Rf=0.39), whereas the structure of SuSyII was consisted of identical 63 kDa monomers (Rf=0.31) (fig. 4). Figure 3. Calibration curve for molecular weight estimation of chromatographically separated proteins on Sephadex G-150 gel Kav – coefficient of partitioning, A – cytochrome c (12.3 kDa), B – trypsin (23.8 kDa), C – ovoalbumin (45.0 kDa), D – bovine serum albumin (66.0 kDa), E – myosine (205.0 kDa) Vol. 54 No 3 2008 H. Sytykiewicz, P. Czerniewicz, B. Leszczyński 46 Figure 4. Electropherograms of sucrose synthase fractions isolated from bird cherry leaves separated with the use of SDS-PAGE The sucrose synthase has been isolated and highly purified from several organs and tissues of different plant species, i.e. wheat, cucumber, Japanese pear fruit, soybean nodules, sugarcane, tobacco and sycamore cells [5-8, 15]. Many authors emphasize that number of SuSy isoenzymes occurring within relevant subcellular compartments or tissues depends on numerous factors [16-18]. One of the most important variables affecting the sucrose synthase profile within cells is maturing and physiological modifications of plant organs. According to Klotz et al. [11] activity of SuSy isoenzymes was correlated with phase of ontogenetic development of sugar beet. SuSyII showed its biochemical activity only in mature organs, whereas SuSyI catalysed reactions of sucrose synthesis and cleavage mostly within growing tissues. The DNA of Arabidopsis thaliana (L.) encodes six SuSy-like genes with distinct expression, down- or up-regulated by various environmental stressors such as anoxia, dehydration, cold treatment and wounding [19-22]. The two Molecular characteristics of sucrose synthase isolated from bird cherry leaves 47 SuSy isoformes of analysed enzyme which were distinguished in presented studies have also been found in several eudicotyledons, i.e. potato, carrot, Japanese pear fruit and BY-2 tobacco heterotrophic cells [5, 6, 8]. Matic et al. [6] suggested that SuSyI is more involved in channeling metabolites into glycolysis, and SuSyII is more active during cell wall biosynthesis. Differences in net charges or charge distribution within isoenzymes implicate variable biochemical functions they act. In maize, the SS1 isoform was involved in starch synthesis and dominated in the endosperm, whereas the SS2 isoenzyme was active in providing substrate for cell wall formation and glycolysis [6]. Application of gel filtration with the use of Sephadex G-150 bed for native SuSy molecular mass estimation allowed to determine that both isoenzymes possessed comparable molecular weight (SuSyI – 200 kDa, SuSyII – 180 kDa). Review of published experimental data indicate that molecular weight of this enzyme is placed in the range of 320 kDa (Beta vulgaris L., Daucus carota L.) to 540 kDa (Cucumis sativus L., Ipomoea batatas L.) [20]. Depending on ionic strength of buffer solution, the enzyme may form aggregated structures. Explanation of high molecular mass of SuSy is also S170 phosphorylation that promotes the formation of complex dimensional forms. Subunit composition of the sucrose synthase was evaluated using the SDS-PAGE technique under denaturing conditions. This method allowed to separate and reveal SuSy subunits within polyacrylamide gel. Electropherograms of the analysed isoenzymes isolated from bird cherry tissues indicated that their structure was probable composed of three subunits. Published data referring to analysed enzyme extracted from different plant species proved that SuSy may possess monomers with molecular mass reaching up to 94 kDa and form complex three-dimensional structures, mostly tetrameric or oligomeric [5, 7, 8, 20]. Klotz et al. showed that SuSyI isolated from Beta vulgaris L. roots was consisted of two 84 kDa subunits and two 86 kDa subunits (heterotetramer), whereas sucrose synthase isoform II (SuSyII) was composed of four subunits of 86 kDa (homotetramer) [11]. The proposed protocol of isolation and purification of two SuSy isoforms extracted from bird cherry leaves enables elucidating their more extensive kinetic properties (i.e. nature of inhibition in sucrose synthesis and cleavage reactions) and also evaluating metabolic mechanisms regulating isoenzymes’ activity. CONCLUSIONS 1.Two isoforms of SuSy have been isolated from bird cherry leaves with high yield and purified to near-homogeneity state. 2.The relative molecular mass of the native isoenzymes was estimated to be 200 and 180 kDa, respectively. 3.Isoform SuSyI was a heteromer contained two different subunits (57.5 and 52.8 kDa), whereas the structure of SuSyII was consisted of identical 63 kDa subunits (homomer). Vol. 54 No 3 2008 H. Sytykiewicz, P. Czerniewicz, B. Leszczyński 48 4.The dimensional structure of the sucrose synthase isoenzymes is probably composed of three subunits. REFERENCES 1.Ren G, Healy RA, Horner HT, James MG, Thornburg RW. Expression of starch metabolic genes in the developing nectarines of ornamental tobacco plants. Plant Sci 2007; 173:621-37. 2. Hirose T, Scofield GN, Terao T. An expression analysis profile for the entire sucrose synthase. Plant Sci 2008; 174:534-43. 3.Rosales MA, Rubio-Wilhelmi MM, Castellano R, Castilla N, Ruiz JM, Romero L. Sucrolytic activities in cherry tomato fruits in relation to temperature and solar radiation. Sci Horticult 2007; 113:244-49. 4.Etxeberria E, Gonzalez P. Evidence for a tonoplast-associated form of sucrose synthase and its potential involvement in sucrose mobilization from the vacuole. J Exp Bot 2003; 54:1407-14. 5.Tanase K, Yamaki S. Purification and characterization of two sucrose synthase isoforms from Japanese pear fruit. Plant Cell Physiol 2000; 41:408-14. 6. Matic S, Åkerlund H-E, Everitt E, Widell S. Sucrose synthase isoforms in cultured tobacco cells. Plant Physiol Biochem 2004; 42:299-306. 7.Barratt DH, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol 2001; 127:655-64. 8.Römer U, Schrader H, Gunther N, Nettelstroth N, Frommer WB, Elling L. Expression, purification and characterization of recombinant sucrose synthase 1 from Solanum tuberosum L. for carbohydrate engineering. J Biotechnol 2004; 107:135-49. 9. Leather SR. Biological flora of the British Isles. Prunus padus L. J Ecology 1996; 84:125-32. 10.Röhring H, John M, Schmidt J. Modification of soybean sucrose synthase by S-thiolation with ENOD40 peptide A. Biochem Bioph Res Co 2004; 325:864-70. 11.Klotz KL, Finger FL, Shelver WL. Characterization of two sucrose synthase isoforms in sugar beet root. Plant Physiol Biochem 2003; 41:107-15. 12. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680-85. 13. Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 1944; 153:375-80. 14. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265-75. 15. Lutfiyya LL, Xu N, D’Ordine RL, Morrell JA, Miller PW, Duff SM. Phylogenetic and expression analysis of sucrose phosphate synthase isozymes in plants. J Plant Physiol 2007; 164:923-33. 16. MacGregor EA. Possible structure and active site residues of starch, glycogen and sucrose synthases. J Protein Chem 2002; 21:297-306. 17.Klotz KL, Haagenson DM. Wounding, anoxia and cold induce sugar beet sucrose synthase transcriptional changes that are unrelated to protein expression and activity. J Plant Physiol 2008; 165:423-34. 18.Watkinson JI, Hendricks L, Sioson AA, Heath LS, Bohnert HJ, Grene R. Tuber development phenotypes in adapted and acclimated, drought-stressed Solanum tuberosum ssp. andigena have distinct expression profiles of genes associated with carbon metabolism. Plant Physiol Biochem 2008; 46:34-45. 19.Zabalza A, Gálvez L, Marino D, Royuela M, Arrese-Igor C, González EM. The application of ascorbate or its immediate precursor, galactono-1,4-lactone, does not affect the response of nitrogen-fixing pea nodules to water stress. J Plant Physiol 2008; 165:805-12. 20. www.brenda.com 21. Marraccini P, Geromel C, Ferreira LP, Pereira LFP, Vieira LGE, Leroy T, Pot D, Mazzafera P. Sucrose metabolism during fruit development of Coffea racemosa. Ann Appl Biol 2008; 152:179-87. 22. López M, Herrera-Cervera JA, Iribarne C, Tejera NA, Lluch C. Growth and nitrogen fixation in Lotus japonicus and Medicago truncatula under NaCl stress: Nodule carbon metabolism. J Plant Physiol 2008; 165:641-50. Molecular characteristics of sucrose synthase isolated from bird cherry leaves 49 CHARAKTERYSTYKA MOLEKULARNA SYNTAZY SACHAROZY IZOLOWANEJ Z LIŚCI CZEREMCHY ZWYCZAJNEJ Hubert Sytykiewicz*, Paweł Czerniewicz, Bogumił Leszczyński Katedra Biochemii i Biologii Molekularnej Akademia Podlaska ul. Prusa 12 08-110 Siedlce *autor, do którego należy kierować korespondencję: tel./faks: +4825 6431367, e-mail: [email protected] Streszczenie W przeprowadzonych badaniach dokonano charakterystyki molekularnej częściowo oczyszczonej syntazy sacharozy (NDP-glukoza: D-fruktoza 2-α-D-glukozylotransferaza, EC 2.4.1.13), ekstrahowanej z liści czeremchy zwyczajnej (Prunus padus L.). Syntaza sacharozy została oczyszczona w wysokim stopniu przy wykorzystaniu czteroetapowej procedury obejmującej precypitację siarczanem amonu, dializę, filtrację żelową na złożu Sephadex G-150 i chromatografię jonowymienną na złożu DEAE-celuloza. Analizowany enzym występował w postaci dwóch izoenzymów (SuSyI i SuSyII). Względny ciężar cząsteczkowy natywnych form analizowanego enzymu wynosił odpowiednio 200 i 180 kDa. Izoforma SuSyI zawierała w swym składzie dwie różne podjednostki (57,5 i 52,8 kDa), podczas gdy struktura SuSyII składała się z monomerów o ciężarze cząsteczkowym 63 kDa. Przeprowadzone badania wskazują, że obydwie izoformy SuSy zawierały po trzy podjednostki. Słowa kluczowe: syntaza sacharozy, Prunus padus, ciężar cząsteczkowy, skład podjednostkowy Vol. 54 No 3 2008