* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Understanding oxidative stress and antioxidant functions in order to
Gaseous signaling molecules wikipedia , lookup
List of types of proteins wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Gene regulatory network wikipedia , lookup
Glutathione wikipedia , lookup
Plant nutrition wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biochemical cascade wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Plant breeding wikipedia , lookup
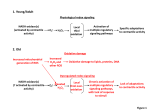
Plant Physiology Preview. Published on November 2, 2010, as DOI:10.1104/pp.110.166181 Running Title: Protection from Oxidative Stress. Corresponding author: Christine H. Foyer Centre of Plant Science Research Institute of Integrative and Comparative Biology Faculty of Biological Science University of Leeds Leeds LS2 9JT UK Tel +44 (0)113 343 1421; Fax +44(0)113 323 3144 Email: [email protected] Research area: Update for Special Issue “Enhancing photosynthesis” 1 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Copyright 2010 by the American Society of Plant Biologists Update: Understanding oxidative stress and antioxidant functions in order to enhance photosynthesis Christine H. Foyer1 and Shigeru Shigeoka2 1 Centre for Plant Sciences, Faculty of Biology, University of Leeds, Leeds, LS2 9JT, UK. 2 Department of Advanced Bioscience, Kinki University, Nakamachi, Nara 631-8505, Japan 2 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Footnotes: Part of this work was supported by EU funded project on Chloroplast Signals: COSI (ITN-GA-2008-215174), and part by a Grant-in-aid for Scientific Research (A) (S.S. :22248042) from the MEXT, JAPAN. Author to whom correspondence should be addressed: Email: [email protected] Tel +44 (0)113 343 1421; Fax +44(0)113 323 3144 The author(s) responsible for distribution of materials integral to the findings presented in this article in accordance with policy described in the instructions for Authors (www.plantphysiol.org) is: Christine H. Foyer ([email protected]) 3 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Photosynthesis is a well established source of reactive oxygen species (ROS) in plants. The photosynthetic transport chain operates in an aerobic environment and thus regulatory systems are required to minimize ROS production. An efficient antioxidant network is also essential in order to process ROS effectively and to maintain intracellular ROS pools at low levels. The importance of the antioxidant network in maintaining high rates of photosynthesis has been demonstrated in many studies using molecular genetics approaches to either disable antioxidant enzymes or boost their activities or the abundance of low molecular weight molecular antioxidants such as ascorbate (AsA) and reduced glutathione (GSH). However, such studies have often failed to take into account the photosynthetic production of reductants and oxidants within the context of cellular redox homeostasis and redox signaling. Our understanding of the interactions of redox signaling pathways with photosynthesis has greatly increased in recent years and this provides new opportunities for enhancing photosynthesis particularly under conditions that favor cellular oxidation. Here we consider current concepts of oxidative stress and oxidative signaling in relation to photosynthesis and whether ROS scavenging systems have evolved to be “leaky” in order that ROS can function as a useful signaling system. Oxidative Stress and the role of reactive oxygen species The field of redox biology has recently witnessed a dramatic reappraisal of the importance of ROS. Due to the reactivity of ROS and because they are unavoidable by-products of oxygenic photosynthesis, only the more negative aspects of ROS generation are often considered in relation to observations. Thus, light-driven ROS production is generally described as harmful because it can cause irreversible damage to photosynthetic components. It is thus generally often suggested that ROS production should be minimized at all costs. However, despite their potential for causing harmful oxidations, it is now well established that ROS are also powerful signaling molecules, which are involved in the control of plant growth and development as well as priming acclimatory responses to stress stimuli (Jones, 2006; Foyer and Noctor, 2005 a, b; 2009). Nevertheless, in many studies 4 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. involving photosynthetic ROS generation, the signaling function of these powerful metabolites is largely ignored and interpretations are all too frequently based solely on the notion that ROS exert their principal effects through chemical toxicity and their abilities to cause damage. Within this context the term “oxidative stress” has become largely synonymous with “oxidative damage”, to cellular components, particularly in situations where oxidative inactivation exceeds that of repair or replacement. Furthermore, it is often suggested that the accumulation of damaged cellular components and associated loss of function leads to cell death but the mechanisms that cause cell death in this case are generally vague or undefined. Relatively few studies to date have considered photosynthetic ROS generation in the context of the light-driven production of powerful signaling molecules, whose abundance provides essential information to the cell concerning imbalances between energy-generating and energy-utilizing processes in the photosynthetic electron transport (PET) system. Within this context, increased ROS production leading to enhanced oxidation in high light may be considered to be a powerful signal that not only decreases photosystem II (PSII) activity but also stimulates gene expression, particularly with regard to acclimation and defense genes. While concepts of ROS function in signaling or damage in photosynthesis are largely irrelevant to the description of the basic biochemical mechanisms by which ROS oxidize cellular components, the philosophical choice between ‘damage’ and ‘signaling’ in data analysis, remains crucial to the evaluation of the physiological significance of these mechanisms. Oxidant production and the regulation of photosynthesis Oxygenic photosynthesis is a dynamic and flexible process that powers life on earth, in which water oxidation on the lumen side of PSII is an indispensable step. The light driven PET system drives electrons from water through to NADP, generating the proton gradient that facilitates ATP synthesis. Intriguingly, a key feature of PSII is its vulnerability to light-induced damage, which is considered to be a consequence of the production of singlet oxygen (Vass and Cser, 2009) in the PSII reaction centre leading to irreversible oxidation 5 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. of the D1 protein (Krieger-Liszkay et al., 2008). This sensitivity means that the PSII reaction centre has to be re-built about once every 30 min even under relatively low irradiances. The damage and repair process thus occurs under all light intensities. A limitation of the PET system only occurs when the rate of damage exceeds that of repair and this makes a major contribution to the processes called photoinhibition (Vass and Aro, 2008). In many circumstances where the rate of damage is fast such as at high light intensities, the rate repair is also rapid (Ohad et al., 1984) and so a high level of PSII activity can be maintained. It is widely accepted that efficient regulation of PET serves to minimize the production of singlet oxygen at PSII, as well as the generation of superoxide and H2O2, which occurs predominantly on the reducing side of photosystem I (PSI) (Genty and Harbinson, 1996). Within this context, reversible decreases in the efficiencies of both photosystems are intrinsic to the regulation of light use by photosynthesis (Genty and Harbinson, 1996). Inherent limitations on the capacity for electron transport through the cytochrome b6/f (cytb6f) complex favor over-reduction of PSII, which exacerbates PSII turnover (Vass and Cser, 2009; Genty and Harbinson, 1996). Of the strategies that can be employed to protect PSII under conditions of limiting PET capacity, the most important is non-photochemical quenching (NPQ), which dissipates the excitation energy of chlorophyll a molecules safely as heat (Niyogi et al., 1998). The majority of electron flow follows a linear, non-cyclic route from water through PSII, the cytb6f complex and PSI to NADP, leading to the generation of both NADPH and ATP. However, the operation of a cyclic electron flow pathway around PSI provides a mechanism whereby ATP production can be increased relative to NADPH. Moreover, the operation of cyclic electron flow is also considered to prevent over-reduction of the acceptor side of PSI (Munekage et al., 2008). This is important because superoxide and H2O2 are generated by PET components on acceptor side of PSI. Cyclic electron flow may also help to limit singlet oxygen production at PSII because it enhances protonation in the 6 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. lumen, which triggers protective NPQ mechanisms (Rumeau et al., 2007). The exact contribution of the linear and cyclic pathways to overall electron flow depends on cell type and environmental conditions. However, in C3 leaves under optimal growth conditions, the major function of cyclic electron flow around PSI is considered to be the augmentation of ATP production relative to NADPH in order to balance the energy budget of the chloroplast. While many fundamental questions remain concerning the components involved in cyclic electron flow and the extent to which this pathway operates in C3 plants, there is a general consensus of opinion that cyclic electron flow is an essential component of the repertoire of chloroplast mechanisms that serve to co-ordinate energy metabolism and balance redox status. A further mechanism of regulation for photosynthesis that prevents over-reduction of the PSI acceptor side and the chloroplast stroma is the “malate valve” system, which transfers reducing equivalents to the cytosol (Scheibe et al., 2005). This pathway, which is activated by the thioredoxin (TRX)-regulated activation of chloroplastic NADP-dependent malate dehydrogenase, is an essential component for the stromal redox homeostasis network because it allows the export of excess reducing power and thus relieves electron pressure in the chloroplast. In this way, the regulation of metabolite distribution can be used to balance cellular redox status in order to achieve metabolic and photosynthetic control. However, to date little attention has been paid to how the regulated distribution of other metabolites, particularly antioxidants, may alter the stromal redox homeostasis network and affect the regulation of photosynthesis. The antioxidant network of chloroplasts Photosynthesis is an important source of cellular oxidants (Foyer and Noctor, 2003). Even under optimal conditions, the calculated rate of H2O2 formation by the PET chain in the chloroplasts during photosynthesis in C3 leaves is nearly as high (4 μmol.m–2.s–1) as the amount produced in the peroxisomes as a result of the glycolate oxidation in the 7 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. photorespiratory pathway (10 μmol. m–2.s–1 ; Foyer and Noctor, 2003). While it is often stated that the amount of H2O2 in chloroplasts can increase by several orders of magnitude during stress, such notions must be viewed with caution because of the inherent complexities of obtaining accurate measurements of H2O2 contents in intact tissues and isolated organelles (Queval et al., 2008). Current methods do not allow accurate estimations of the H2O2 concentration of the stroma under either optimal or stress conditions. Regardless of any uncertainties about absolute values for H2O2 levels in photosynthetic tissues, it has long been recognized that H2O2 is a potent inhibitor of photosynthesis because even at low concentrations (10 μM) it can inhibit CO2 fixation by 50% because of the oxidation of the thiol-modulated enzymes of the Calvin cycle. Therefore, the balance between ROS-production and ROS-scavenging in chloroplasts is delicate and must be strictly controlled. AsA and GSH are the most abundant and best characterized water-soluble antioxidants in plants, and they accumulate to millimolar concentrations in chloroplasts. Although these metabolites scavenge ROS separately, they have long been considered to function together in the AsA/GSH cycle (Foyer and Halliwell, 1976) and water-water cycle (Asada, 1999) to metabolize H2O2 (Figure 1) and to dissipate excess excitation energy in chloroplasts. The reduction of ground state molecular oxygen to superoxide on the acceptor side of PSI in the Mehler reaction is the first step of a series of reactions that together has been called “the water-water cycle” (Asada, 2000). This incorporates superoxide dismutase (SOD), AsA peroxidase (APX) and the AsA-GSH cycle (Figure 1) in a mechanism that builds up ΔμH+ and facilitates ATP formation at the expense of NADPH and reductant (Asada, 1999). APX utilizes AsA as a specific electron donor to reduce H2O2 to water with the concomitant generation of monodehydroascorbate (MDA), a univalent oxidant of AsA. While MDA is spontaneously converted to AsA and dehydroascorbate (DHA), it is also rapidly reduced to AsA by the action of a NAD(P)H-dependent MDA reductase (MDAR). DHA reductase (DHAR) utilizes GSH to reduce DHA and thereby regenerate AsA. GSH is then regenerated from glutathione disulfide (GSSG) by the action of glutathione reductase 8 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. (GR) using NAD(P)H. While different APX and SOD isoforms are located in the stroma and thylakoid membrane, the chloroplastic GR and DHAR enzymes are localized in the stroma. A characteristic property of APX, particularly the chloroplastic APX forms, is their susceptibility to oxidative inactivation in the absence of AsA (Ishikawa and Shigeoka 2008). Under low AsA concentrations (less that 20 µM) the activity chloroplastic APXs is rapidly lost in the presence of H2O2, with a half-inactivation time of less than 30 sec. In contrast, the cytosolic and peroxisomal APXs only lose activity after more than an hour. The chloroplastic APXs are the primary targets for inactivation if chloroplast ascorbate accumulation is impaired. Depletion of chloroplast AsA and inactivation of chloroplast APXs have therefore been considered as limitations of photosynthetic efficiency in stress conditions (Ishikawa and Shigeoka, 2008) and therefore potential targets for improvement. In addition to the AsA-GSH cycle, other proteins that are important in chloroplast ROS detoxification are peroxiredoxin (PRX, particularly 2-CysPrx and PrxQ), glutathione peroxidase (GPX), sulfiredoxin and cyclophilin, which function together with Trx and Trx-like proteins in the chloroplasts (Dietz 2011). The thiol-based catalytic mechanism used by PRX to reduce H2O2, consists of a peroxidative reduction, followed by regeneration that can involve a variety of electron donors such as TRX, glutaredoxin (GRX), cyclophilins, GSH and AsA. GPXs can use both GSH and TRX as reducing substrates and they can detoxify lipid peroxides as well as H2O2 (Herbette et al., 2002). Because TRX is a more efficient substrate than GSH and their high rates of TRX-dependent peroxidase activity, plant GPXs have been assigned as to the PRX protein family (Dietz et al., 2006). In the GPX system, the regeneration of reduced TRX is linked to the PET chain through either ferredoxin (Fd)-TRX reductases (FTR) or NADPH-dependent TRX reductases (NTR). While the catalytic rates of plant GPXs and their affinities for H2O2 are rather low compared to APX, the PRX and GPX pathways provide an alternative pathway to the water-water cycle in the light (Figure 1), particularly if the AsA-GSH cycle is impaired. However, lipid peroxides are also efficient substrates for the chloroplast GPXs. The detoxification of lipid peroxides, which exacerbate the lipid 9 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. peroxidation cascade reactions, may be as equally important as H2O2 detoxification in terms of maintaining optimal photosynthetic functions in the light. AsA-GSH cycle and the PRX-dependent detoxification pathways may be equally important in vivo and serve interfacing functions. Our view is that the two detoxification pathways are tailored to suit specific niches in defense metabolism and their relative importance probably varies according to the prevailing environmental conditions. The observation that chloroplastic mDHARs and APXs are enhanced in antisense Arabidopsis plants with suppressed chloroplastic-located PRX (Baier et al., 2000), suggests that regulatory compensation mechanisms exist between the pathways, so that one pathway may be enhanced to compensate for losses in the other pathway. Such observations of interactions between the AsA-GSH pathway and the PRXs demonstrate cross-talk between the individual ROS-metabolizing pathways of the chloroplasts. The AsA-GSH pathway has a higher specificity for H2O2 and the chloroplast APX has higher activities than PRXs but the PRXs have a broad specificity of towards lipid peroxides and/or reactive nitrogen species, such as nitric oxide (NO). For example, values for the catalytic efficiencies (Kcat/KM ; L.mol-1.s-1) for plant 2-CysPrx with H2O2 range between 2.5.104 to 1.8.103 with a value of 7.3.103 with tBOOH (Dietz, 2011). In comparison, the value for AsA-dependent H2O2 reduction is 0.9.106 L. mol-1.s-1 (Asada, 1999). Like ROS, NO is an important plant signaling molecule. NO reacts rapidly with the superoxide anion to produce peroxynitrite. It is likely that peroxynitrite is produced in chloroplasts, where it may fulfill signaling functions. Moreover, in marked contrast to the situation in animals, peroxynitrite does not appear to be toxic to plants, which appear to function without problem even in the presence of high levels of this metabolite. A key feature of the regulation of NO metabolism is its reaction with GSH to form nitrosoglutathione (GSNO), which can then react with other thiols to form nitrosothiols (Lindermayr et al., 2005). GSNO serves as a storage pool of NO and it probably also fulfils as yet unknown signaling functions. Like glutathionylation, protein S-nitrosylation can 10 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. modify both protein function and activity (Noctor et al., 2011) While the AsA/GSH cycle operates largely within the stroma, it is also linked to the functions of a hydrophobic antioxidant, α-tocopherol (Toc), which is maintained in its reduced form by AsA. Carotenoids and tocopherols are the most abundant groups of lipid-soluble antioxidants in chloroplasts (DellaPenna and Pogson, 2005). Toc is accumulated to high concentrations in chloroplasts, where it serves to prevent lipid peroxidation by removal of singlet oxygen and lipid peroxyl radicals (Krieger-Liszkay et al., 2008). The resultant tocopheroxyl radicals are reduced back to Toc by AsA and the action of the AsA/GSH cycle. The Arabidopsis vte1 mutants that are deficient in Toc show a significant accumulation of AsA and GSH. In contrast, VTE1-overexpressing plants, which accumulate Toc, have lower AsA and GSH levels (Kanwischer et al., 2005). Such observations provide further evidence of the close relationships that exist between chloroplast antioxidants, which compensate for each other. Moreover, these data not only demonstrate the high degree of interaction between the chloroplast antioxidant pathways but they also suggest that there are multiple sites of reciprocal control. However, while the biosynthetic pathways for AsA (Smirnoff et al., 2001), GSH (Mullineaux and Rausch, 2005) and Toc (DellaPenna and Pogson, 2005) are now well established, much remains to be understood regarding how the synthesis and accumulation of these essential antioxidants is controlled and regulated in a coordinated manner. The factors that control the intracellular partitioning of metabolites between the different compartments of the cell and the antioxidant transport systems are of particular importance to the overall regulation of photosynthesis and its effective operation over a wide range of environmental conditions. While recent years have witnessed an increase in our understanding of the roles of ROS in the signaling systems that coordinate antioxidant gene expression, very little information is available to date on how the low molecular weight antioxidants participate in this control. Genetic manipulation of antioxidant defenses of chloroplasts 11 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. A wide range of strategies, including molecular genetics and genetic engineering approaches, have been used to enhance the tolerance of plants to abiotic stress (Mittler and Blumwald, 2010). Many attempts have been made over the last two decades to protect photosynthesis against stress-induced inhibition by manipulation of component antioxidant enzymes and extensive literature exists showing that enhancing the capacity of the water-water cycle through genetic engineering, including the overexpression of SOD, GR, and DHAR, can improve the tolerance of plants to abiotic stress (Logan et al., 2006). Overall, the enhancement of chloroplast antioxidant defenses has proved to be one of the most effective ways of protecting plant cells from abiotic stress (for example, Ishikawa and Shigeoka, 2008; Chang et al., 2009). Taken together, studies that demonstrate that an enhancement of antioxidant capacity gives added protection to photosynthesis illustrate the point that there is not an excess capacity for ROS destruction in the chloroplasts. Rather, such findings suggest that the capacity for ROS detoxification in the chloroplasts is balanced with regard to ROS production. This is perhaps surprising given the susceptibility of the photosynthetic processes to oxidative inactivation. However, if one considers that ROS generated in photosynthesis participate in a redox signaling cascade then the importance of regulation of both ROS abundance and the life-time of the ROS signal becomes apparent. The available data suggest that ROS detoxification pathways are not present in amounts that would totally remove all the ROS from the cellular environment. Rather, there appears to be a level of co-ordination between ROS producing- and ROS-metabolizing processes that allows a pool of ROS to persist in the cellular environment. This co-ordinate regulation allows increases in the available ROS pool to occur during stress, presumably for signaling purposes. The size of the ROS signaling pool will depend on the relative rates of ROS production and destruction, the latter of which can be finely tuned with regard to enzyme activation and gene expression. In this context, the balance between oxidant production and removal by the antioxidant system would essentially determine the strength and lifetime of the ROS 12 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. signal, as illustrated in Figure 2. In addition, the oxidation of some proteins could trigger altered pathways of gene expression while oxidation of other proteins such as those in PSII reaction center might further provide a mechanism that would enable more rapid adjustment of PSI and PSII stoichiometries to changing environmental conditions. Oxidative modifications to specific amino acids is a widespread and generally accepted mechanism of marking proteins for proteolysis, Within the context of the “oxidative stress-induced damage”, hypothesis, the added protection of photosynthesis during stress that has been obtained by enhancing chloroplast antioxidant network provides evidence that it is possible to mitigate potentially harmful reactions caused by enhanced oxidative load (Logan et al., 2006). For example, transgenic tobacco and Arabidopsis plants overexpressing tAPX in the chloroplasts exhibited enhanced tolerance to high light and paraquat treatment, a combination that is designed to maximize photooxidative stress (Ishikawa and Shigeoka, 2008). Similarly, transgenic tobacco overexpressing KatE, a gene encoding an E. coli catalase (CAT), in the stroma, resulted in a greatly enhanced tolerance to photo-oxidative stress (Miyagawa et al., 2000). In this case the enhanced tolerance was explained in terms of compensation for inactivation of chloroplastic APXs by KatE. The physiological significance of the high susceptibility of chloroplastic APXs to inactivation by ROS remains a mystery if one considers only the potential for increased ROS-induced damage. However, from a signaling perspective, the inactivation of chloroplastic APXs may have a regulatory role in facilitating redox signaling pathways under conditions of high ROS production or oxidative stress. Ectopic expression of useful genes from cyanobacteria into the chloroplasts of higher plants has also proved to be effective at enhancing tolerance to abiotic stress. For example, the GPX-like proteins of Synechocystis PCC 6803 are able to reduce unsaturated fatty acid hydroperoxides using NADPH as an electron donor, but not GSH and TRX. The expression of Synechocystis GPX in Arabidopsis chloroplasts resulted in a suppression of photoinhibition and an increased tolerance to photooxidative stress (Gaber et al., 2004). 13 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Moreover, the expression of a cyanobacterial flavodoxin, which is iso-functional with Fd, in chloroplasts enhanced tolerance to a range of abiotic stresses (Zurbriggen et al., 2008). Flavodoxins are considered to prevent electron misrouting and thus contribute to the stress tolerance by reducing the probability of ROS formation (Zurbriggen et al., 2008). The evidence described above confirms the importance of the antioxidative enzymes in chloroplasts, and the value of genetically-enhanced antioxidants and other defense compounds as a mechanism for stabilizing photosynthesis in stress situations. Other metabolites can also serve protective functions in chloroplasts. For example, oligosaccharides such as galactinol and raffinose, which often accumulate to high concentrations, can also act as antioxidants and function as osmoprotectants in plant cells. However, they differ from AsA, GSH and tocopherol as such molecules are generally destroyed as a result of oxidation and they cannot be recycled. Transgenic Arabidopsis plants overexpressing galactinol synthases (GolS1 and GolS2), key enzymes in the synthesis of galactinol and raffinose, had increased levels of these oligosaccharides, and enhanced tolerance to enhanced oxidation caused by exposure to paraquat, chilling and osmotic stress (Nishizawa et al., 2008). Thus, these oligosaccharides can function to protect plant cells from oxidative damage (Nishizawa et al., 2008). Similarly, it is well established that the enhanced biosynthesis of glycinebetaine, an osmoprotectant, increases the tolerance of plants to various types of abiotic stress (Chen and Murata, 2008). While glycinebetaine does not scavenge ROS directly, glycinebetaine synthesis produces H2O2 that activates ROS-scavenging enzymes and thus mitigates against oxidation. The accumulation of glycinebetaine appears to be more effective in the chloroplasts than in the cytosol, suggesting its ability to protect the photosynthetic machinery (Chen and Murata, 2008). Antioxidants and redox signaling in chloroplasts Extensive evidence now points to important roles for oxidative and reductive signaling in the regulation of photosynthesis, with antioxidants such as AsA and GSH having multiple 14 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. signaling functions (Foyer and Noctor, 2009). While manipulation of the antioxidant network has repeatedly shown that depletion of individual antioxidant components has a negative effect on photosynthesis, a number of studies have demonstrated that our original hypotheses concerning ROS and antioxidants are simplistic and in some cases naïve. For example, while depletion of either CAT or cytosolic APX has a general negative impact, tobacco plants that are deficient in both CAT and cytosolic APX show a less marked phenotype than the parent lines (Riszhsky et al., 2002). Thus, whether the functions and action of ROS and antioxidants associated with photosynthesis are often most usefully described within the framework of ‘damage’ or ‘signaling’ these metabolites are part of the much more complex cellular system that governs cell fate in terms of triggering programmed cell death or enhancing defenses to ensure survival. Thanks to the pioneering work of Klaus Apel and his colleagues (for example Laloi et al., 2007), it is now well established that singlet oxygen generated by photosynthesis triggers a genetically programmed suicide pathway that leads to cell death (Figure 2). However, it has only more recently been shown that metabolically generated H2O2 also causes cell death through a genetically programmed pathway that requires salicylic acid (Mhamdi et al., 2010) and is modulated by myo-inositol (Chaouch and Noctor, 2010). Manipulation of these signaling pathways thus provides a novel mechanism to protect photosynthesis and leaf cells from the cell death triggered by metabolically generated ROS. While superoxide production in the Mehler reaction is undoubtedly of crucial importance to the chloroplast redox system, the production of H2O2 during the glycolate oxidase reaction of photorespiration is often the most significant in terms of overall oxidant production in C3 plants (Foyer and Noctor, 2003). Despite the high rates of H2O2 generation in the peroxisomes of C3 plants in air, cat2 mutants that are deficient in the major leaf CAT isoform do not accumulate H2O2. Rather, the total pool size is greatly increased and there is a low GSH/GSSG ratio (Queval et al., 2007; 2009). This has lead to the hypothesis that many of the responses of plant cells to enhanced oxidation including changes in gene expression caused by metabolically-generated H2O2 are transmitted through changes in the 15 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. GSH pool rather than by H2O2 per se (Queval et al., 2010). Moreover, unlike the double mutants lacking both CAT and cytosolic APX, the double mutants lacking both CAT and cytosolic GR have a more severe phenotype than the cat2 single mutants (Mhamdi et al., 2010). This finding may support the view that GR and GSH participate in AsA-independent H2O2 metabolism as described above, as well as in the AsA-GSH pathway. While AsA and GSH act alongside CAT in high capacity redox-homeostatic H2O2-processing pathways, these antioxidants have discrete and specific functions in photosynthesis and associated redox signaling (Foyer and Noctor, 2009). In addition to the AsA-GSH cycle, AsA has several other important roles in photosynthesis. It is a cofactor for violaxanthin de-epoxidase, an enzyme required for NPQ formation, and it participates in the abscisic acid-mediated regulation of stomatal closure. Finally, it can strongly influence the expression of both nuclear and chloroplast genes encoding photosynthetic components (Kiddle et al., 2003). Given the many roles of AsA in the regulation of photosynthesis it is hardly surprising that AsA-deficient plants show zeaxanthin depletion and an inhibition of photosynthesis when exposed to high light (Muller-Moule´ et al., 2004). Involvement in chloroplast to nucleus retrograde signaling pathways The chloroplast ROS, AsA and GSH pools have direct interactions with the photosynthetic machinery and they also participate in signaling pathways that enable photosynthesis to acclimate to environmental change. Long-term acclimation to high light is known to involve increases in antioxidants and decreases in light-harvesting antenna size that is likely due to changes in gene expression (Foyer and Noctor, 2009). Moreover, ROS and GSH are considered to act as second messengers in the pathways responsible for high light stress perception and long distance signal transduction (Szechyn´ ska-Hebda, et al., 2010). The high light stress signaling pathways also interact with hormone signaling pathways. For example, abscisic acid (ABA) pathways participate in systemic light signaling, a 16 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. mechanism that appears to involve H2O2 generation in bundle sheath cells (Mullineaux, et al., 2006). Accumulating evidence links AsA, ROS, NO and ABA signaling pathways in the regulation of stomatal closure and at the level of information flow from the chloroplast to the nucleus in order to co-ordinate plastid and nuclear gene expression, in the process called “retrograde”, signaling (Pfannschmidt et al., 2008). In particular, ABA-insensitive-4 (ABI4) has been shown to be important in the regulation of nuclear genes in response to environmental and developmental cues (Koussevitzky et al., 2007). While much remains to be discovered regarding the nature of the crosstalk between the different signaling pathways, it is probable that some degree of control is exerted through the abundance of redox metabolites, as illustrated in Figure 2. Metabolite crosstalk between different pathways involving oxidants and antioxidants not only serves as a mechanism of reciprocal control but it also provides a hub of redox information that readily interfaces with different types of retrograde signals, such as Mg-protophorphyrin IX, that are generated by chloroplasts and that are responsible for the fine-tuning of genes encoding photosynthetic proteins in the nucleus (Pfannschmidt et al., 2008). Intermediates of chlorophyll biosynthesis that produce ROS by photodynamic action are considered to be important in chloroplast-to-nucleus retrograde signaling (Rintamäki et al., 2009). Much of our current understanding of the role of singlet oxygen signaling in chloroplast to nucleus retrograde signaling pathways has been provided by the analysis of Arabidopsis mutants that are deficient in the control of tetrapyrrole biosynthesis, which can be distinguished from the wild-type by the strong protochlorophyllide fluorescence and were hence named fluorescent or flu (Meskauskiene et al., 2001). Changes in the gene expression patterns of the flu mutants relative to the wild type showed that the pathway of singlet oxygen-dependent control of nuclear gene expression was different from that of H2O2 (Laloi et al., 2007). Moreover, singlet oxygen generation in the flu mutant activated a broad range of signaling pathways involved in biotic and abiotic stress responses but this process was found to be essentially antagonistic to the action of H2O2 on certain gene expression patterns (Laloi et al., 2007). Further analysis of the flu mutants revealed that the 17 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. inactivation of a nuclear-encoded plastid protein called EXECUTER1 was sufficient to abolish singlet oxygen–dependent stress responses and attenuate the extent of superoxide-induced up-regulation of nuclear gene expression (Wagner et al., 2004). A second related nuclear-encoded protein, called EXECUTER2, has also implicated in singlet oxygen-dependent control of nuclear gene expression (Lee et al., 2007). Both EXECUTER proteins function in retrograde signaling pathways in the chloroplasts and control singlet oxygen-responsive genes in order to regulate the accumulation of chlorophyll and carotenoids, particularly in high light stress (Lee et al., 2007). In summary, enhancing the activities of antioxidant enzymes and/or the accumulation of low molecular weight antioxidants by genetic manipulation increases tolerance to a variety of stresses, through more efficient removal of ROS. Thus, by making ROS removal more efficient the photosynthetic processes are desensitized to environmental change. Antioxidant systems have evolved not to completely remove ROS but to allow these signals to persist within the cellular environment. The persistence of ROS in stressful environmental conditions serves to limit the capacity of photosynthesis in situations where energy-producing and energy-utilizing processes are not well balanced. Hence, high or enhanced antioxidant capacity can be considered to be generally beneficial because it desensitizes photosynthesis to over-reduction in the PET chain and in some cases such as enhanced water-water cycle activity it can even help alleviate over-reduction. While, there is little information to date concerning how increased antioxidant capacity influences retrograde signaling pathways from the chloroplasts to the nucleus, plants with enhanced antioxidants often show altered local and systemic stress responses. To date, manipulations of plant antioxidant defenses have been largely limited to a small number of enzymes. The elucidation of redox signaling pathways related to both ROS and antioxidants could provide much more useful molecular tools for up-regulation of whole suites of genes with protective functions that could make the photosynthetic system much less susceptible to light and ROS-mediated modifications. 18 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. References Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen species and dissipation of excess photons. Ann. Rev. Plant Physiol. Plant Mol. Biol., 50: 601-639 Baier M, Noctor G, Foyer CH, Dietz KJ (2000) Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. Plant Physiol. 124: 823-832 Chang CC, Slesak I, Jord L, Sotnikov A, Melzer M, Miszalski Z, Mullineaux PM, Parker JE, Karpinska B, Karpinski S (2009) Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol. 150: 670-683 Chaouch S and Noctor G (2010) Myo-inositol abolishes salicylic acid-dependent cell death and pathogen defence responses triggered by peroxisomal H2O2. New Phytol. Doi. 10/111/j.1469-8137.2010.03453.x Chen TH, Murata N (2008) Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 13: 499-505. Dietz KJ (2011) Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Signal. In press. Dietz KJ, Jacob S, Oelze ML, Laxa M, Tognetti V, de Miranda SM, Baier M, Finkemeier I (2006) The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 57: 1697-1709 DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol. 57: 711-738. Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133: 21–25. Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant. 119: 355-364. 19 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Foyer CH, Noctor G (2005a) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28: 1056-1071. Foyer CH, Noctor G (2005b) Redox homeostasis and antioxidant signalling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866-1875 Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: Signaling, acclimation and practical implications. Antioxid. Redox Signal. 11: 862-905 Gaber A, Yoshimura K, Tamoi M, Takeda T, Nakano Y, Shigeoka S (2004) Induction and functional analysis of two reduced nicotinamide adenine dinucleotide phosphate-dependent glutathione peroxidase-like proteins in Synechocystis PCC 6803 during the progression of oxidative stress. Plant Physiol. 136: 2855-2861 Genty B, Harbinson J (1996) Regulation of light utilization for photosynthetic electron transport. In: Baker NR, ed. Photosynthesis and the environment. Kluwer Academic Publications, 67–99 Herbette S, Lenne C, Leblanc N, Julien JL, Drevet JR, Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur. J. Biochem. 269: 2414-2420 Ishikawa T, Shigeoka S (2008) Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 72: 1143-1154. Jones DP (2006) Redefining oxidative stress. Antioxid. Redox Signal. 8: 1865-1879 Kanwischer M, Porfirova S, Bergmüller E, Dörmann P (2005) Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol. 137: 713-723 Kiddle G, Pastori GM, Bernard B, Pignocchi C, Antoniw J, Verrier PJ, Foyer CH (2003) Effects of leaf ascorbate content on defense and photosynthesis gene expression in 20 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Arabidopsis thaliana. Antioxid. Redox Signal. 5: 23-32. Koussevitzky, S., Nott, A., Mockley, T.C., Hong, F., Sachetto-Martins, G., Surpin, M., Lim, J., Mittler, R., and Chory, J. (2007) Multiple signals from damaged chloroplasts converge on a common pathway to regulate nuclear gene expression. Science 316: 715-719. Krieger-Liszkay A, Fufezan C, Trebst A (2008) Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 98: 551-564 Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych IM, Apel K. 2007. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA 104: 672–677 Lee KP, Kim C, Landgraf F, Apel K (2007) EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Nat. Acad. Sci.USA 104: 10270-10275 Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol. 137: 921-930 Logan BA, Kornyeyev D, Hardison J,. Holaday AS (2006) The role of antioxidant enzymes in photoprotection. Photosyn. Res. 88:119-132 Meskauskiene R, Nater M, Goslings D, Kessler F, den Camp RO, Apel K (2001) FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Nat. Acad. Sci.USA 98:12826-12831 Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou JP, Noctor G (2010) Arabidopsis GLUTATHIONE REDUCTASE 1 plays a crucial role in leaf responses to intracellular H2O2 and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 153: 1144-1160 Mittler R, Blumwald E. (2010) Genetic engineering for modern agriculture: challenges and perspectives. Annu. Rev. Plant Biol. 61: 443-462 Miyagawa Y, Tamoi M, Shigeoka S. (2000) Evaluation of the defense system in chloroplasts to photooxidative stress caused by paraquat using transgenic tobacco plants expressing catalase from Escherichia coli. Plant Cell Physiol. 41: 311-320 21 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Mullineaux PM, Rausch T. (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth. Res. 86: 459–474 Munekage YN, Genty B, Peltier G. (2008). Effect of PGR5 impairment on photosynthesis and growth in Arabidopsis thaliana. Plant and Cell Physiology 49: 1688-1698 Mullineaux PM, Karpinski S, Baker NR (2006) Spatial dependence for hydrogen peroxide-directed signaling in light stressed plants. Plant Physiol. 141: 346–350. Nishizawa A, Yabuta Y, Shigeoka S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 147: 1251-1263 Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH (2011) Glutathione. The Arabidopsis Book. (Millar, H. Editor; American Society of Plant Biologists Rockville, MD). In press Ohad I, Kyle DJ, Arntzen CJ (1984) Membrane-protein damage and repair –removal and replacement of inactivated 32- kilodalton polypeptides in chloroplast membranes. J. Cell Biol. 99: 481-485 Pfannschmidt T, Bräutigam K, Wagner R, Dietzel L, Schröter Y, Steiner S,Nykytenko A. 2008. Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Annals of Botany 103, 599–607 Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker, H, Miginiac-Maslow M, Van Breusegem F, and Noctor G (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 52: 640–657 Queval G, Hagar J, Gakie`re, B, Noctor, G (2008) Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J. Expt. Bot. 59: 135–146 22 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Queval G, Thominet D, Vanacker H, Miginiac-Maslow M, Gakière B, Noctor G (2009) H2O2-activated up-regulation of glutathione in Arabidopsis involves induction of genes encoding enzymes involved in cysteine synthesis in the chloroplast. Mol. Plant 2: 344-356 Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inzé D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 32: 329–342 Muller-Moule´ P, Golan T, Niyogi KK (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol. 134: 1163–1172 Rintamäki E, Lepistö A, Kangasjärvi S. 2009. Implication of chlorophyll biosynthesis on chloroplast-to-nucleus retrograde signaling. Plant Signaling Behavior 4, 545–547 Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell and Environment 30, 1041-1051 Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56: 1481-1489 Smirnoff N, Conklin PL, Loewus FA (2001) Biosynthesis of ascorbic acid in plants: a renaissance. Annu Rev Plant Physiol PlantMol Biol 52: 437–467 Szechynska-Hebda M, Kruk J, Gorecka M, Karpinska B, Karpinski S (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22: 1–18 Vass I, Aro E-M (2008) Photoinhibition of photosynthetic electron transport. In: Primary Processes of Photosynthesis: Basic Principles and Apparatus. (Ed. Renger G.) Publ. Roy. Soc. Chem., Cambridge, UK. pp. 393-411 Vass I, Cser K (2009) Janus-faced charge recombinations in photosystem IIphotoinhibition. Trends Plant Sci. 14: 200-205 23 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Wagner D, Przybyla D, op den Camp R, Kim C, Landgraf F, Pyo Lee K, Würsch M, Laloi C, Nater M, Hideg E, Apel K (2004) The genetic basis of singlet oxygen–induced stress responses of Arabidopsis thaliana. Science 306: 1183 – 1185 Zurbriggen MD, Tognetti VB, Fillat MF, Hajirezaei MR, Valle EM, Carrillo N (2008) Combating stress with flavodoxin: a promising route for crop improvement. Trends Biotechnol. 26: 531-537 24 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Legends to figures Figure 1. Ascorbate (AsA-GSH cycle left) and thioredoxin-dependent (TRX/GPX right) pathways of H2O2 removal in chloroplasts. AsA, ascorbate; DHA, dehydroascrobate; DHAR, DHA reductase; Fd, ferredoxin; FNR, ferredoxin NADPH reductase; FTR, ferredoxin thioredoxin reductase; GPX, glutathione peroxidase; GR, glutathione reductase; GRX, glutaredoxin; GSH, reduced glutathione; GSSG, oxidized glutathione; MDA, monodehydroascorbate; MDAR, MDA reductase; NTR, NADPH thioredoxin reductase; PRX, peroxiredoxin; Trx, thioredoxin ; SOD, superoxide dismutase. Figure 2. A simple scheme of the interactions between cellular redox state and photosynthesis within the context of plant stress responses and interactions with hormone signaling pathways. Enhanced oxidation can either lead to either programmed cell death (A) or to enhanced sustainability (B) depending on the intensity of the oxidative signal and the nature of the signaling pathway that is activated. 25 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. AsA GSSG NAD(P)H AsA-dependent pathway DHAR GR Trx-dependent pathway GSH DHA Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Fdred Trxox NADPH H2O MDA APX APX MDAR GPX NTR NADP+ NAD(P)H PRX FTR Fdox NAD(P)+ Trxred H2O2 NAD(P)+ AsA SOD O2- O2 NADP+ Fdred ee- Photosynthetic Electron Transport FNR High Light Environmental Stress Perception Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2010 American Society of Plant Biologists. All rights reserved. Photosynthesis Antioxidants Enhanced Oxidation Hormones Hormones A Cell Death Defense responses B Enhanced Sustainability ROS Cellular Redox Homeostasis