* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Rasha Ageeb Hassan Aly_Rasha
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Aortic stenosis wikipedia , lookup
Electrocardiography wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Myocardial infarction wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Artificial heart valve wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Congenital heart defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
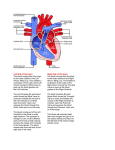
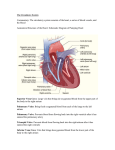
INTRODUCTION Atrial septal defect (ASD) is an opening in the atrial septum permitting the shunting of blood between the atria. There are three major types: ostium secundum, ostium premium, and sinus venosus. Ostium secundum is the most common type (Toyno M, et al., 2008). An isolated secundum atrial septal defect very seldom causes significant symptoms in pediatric patients, regardless of defect size (Abid D, et al., 2013). ASD secondum occurs in 7% of patients with congenital heart disease and is two times more common in females than males. The defect is most often sporadic but may be familial or have a genetic basis such as“Holt-Oram syndrome” (Syamasundar p, et al., 2012). Mitral valve prolapse (MVP) is the most common anomaly of mitral valve, it occurs when one leaflets excessively billows into the left atrium toward the end of systole. Mitral regurgitation (MR) develops in some patients with mitral valve prolapse, particularly those with more significant prolapse (Mansoory MS et al., 2013). The Incidence of mitral valve prolapse in patients with atrial septal defect (ASD) has been reported to be high; this incidence is about 35 % (Suchon E et al., 2004).The reasons for this high incidence has not been completely explained yet. The discussion on the high incidence of MVP in patients with ASD have focused on two hypotheses, one of these is the anatomical co-existence of ASD and MVP as a result of a congenital common connective tissue defect. The other hypothesis is the anomaly occurring in the geometry of the left ventricle due to ASD (Kestelli M et al., 2002). Atrial septal defect closure leads to normalization of left ventricular geometry and in patients with evidence of mitral valve prolapse, is associated with a decrease in the degree of prolapse. (Suchon et al., 2004). Echocardiography is the most useful method of diagnosing a prolapsed mitral valve. Two- and threedimensional echocardiographies are particularly valuable as they allow visualization of the mitral leaflets relative to the mitral annulus. This allows measurement of the leaflet thickness and their displacement relative to the annulus. Thickening of the mitral leaflets >5 mm and leaflet displacement >2 mm indicates classic mitral valve prolapse (Addetia K, et al., 2013). Three-dimensional (3D) echocardiography has recently become a practical reality. It is now practicable to perform 3D echocardiography using transthoracic and transesophageal acoustic windows both in adults and children. An important aspect of 3D echocardiography is its ability to supply accurate quantitative data without the use of geometric assumptions (Bartakian S, et al., 2012). By 3D echocardiography it is possible to visualize the mitral valve en face from either the left atrium or the left ventricle. In volume rendered images looking down in the left atrium, mitral valve prolapse is viewed as a convexity or bulge, and often as bright area when compared with the rest of the mitral leaflet. Looking up in the left ventricle mitral valve prolapse appears as a spoon-like depression. In patients with mitral valve prolapse and mitral regurgitation a crack due to noncoaptation can be identified (Gabriel V`, et al., 2005). AIM OF THE WORK We aimed through this work to identify the effect of ASD closure on the associated MVP and to evaluate the role of 3 D echocardiography in the diagnosis and follow up of this condition. ATRIAL SEPTAL DEFECT Arial septal defect (ASD) is an abnormal opening in the muscular wall separating the left and right upper chambers (atria) of the heart permitting the shunting of blood between the atria. ASDs account for 5% to 10% of all congenital heart diseases , As a group, atrial septal defects are detected in 1 child per 1500 live births and for about 30% of the congenital heart defects diagnosed in adults (Lairakdomrong K, et al., 2013). Figure (1): Atrial septal defect (John J, et al., 2011). Embryology In the early embryo heart, the atria comprise a common chamber. The septum primum, which is the first septum to develop, is an incomplete thin-walled partition in which the anteroinferior free edge is above the atrioventricular canal and becomes lined by tissue derived from the superior and inferior endocardial cushions. Before the resultant interatrial opening (ostium primum) becomes sealed by endocardial cushion tissue, programmed cell death in an area near the anterosuperior aspect of the septum primum creates small cribriform perforations. These perforations coalesce to form a large, second interatrial communication (ostium secundum) maintaining interatrial blood flow. At this time, to the right of the first septum, an anterosuperior infolding of the atrial roof occurs and forms a second septal structure (septum secundum). It expands posteroinferiorly as a thick-walled muscular ridge to form an incomplete partition that overlies the ostium secundum. As atrial septation is accomplished, septum secundum forms the limbus of the fossa ovalis and septum primum forms the valve of the fossa ovalis. The channel for interatrial blood flow through the ostium secundum is known as the foramen ovale (Hidalgo A, et al., 2008). Concurrently with atrial septation, the left horn of the sinus venosus forms the coronary sinus, and the right sinus horn becomes a part of the right atrium. Infolding at the sinoatrial junction forms the right and left venous valves. Whereas the right venous valve is maintained and forms the rudimentary valves of the inferior vena cava (Eustachian valve) and the coronary sinus (Thebesian valve), the left venous valve becomes fused to the superior, posterior, and inferior margins of the fossa ovalis et al., 2012). (Briggs LE, Figure (2) Schematic diagram showing the embryologic sequence of atrial septation. Left, left atrium; right, right atrium; PFO, patent foramen ovale(Briggs LE, et al., 2012). Patent Foramen Ovale The foramen ovale (PFO) represents a normal interatrial communication that is present throughout fetal life, Functional closure of the foramen ovale occurs postnatally as pressure in the left atrium exceeds that in the right atrium. As a result, the valve of the fossa ovalis is pressed against the limbus and forms a competent seal. During the first year of life, fibrous adhesions may develop between the limbus and valve and thereby produce a permanent anatomic seal and an imperforate atrial septum. In 25% to 30% of people, however, anatomic closure does not occur, and a potential interatrial channel persists through which blood or air may shunt whenever pressure in the right atrium exceeds that in the left atrium (Saremi F, et al., 2008). If atrial dilation occurs among individuals with a patent foramen ovale, the limbus may become so stretched that the ostium secundum (valve of the fossa ovalis) may no longer be covered by the limbic ledge. The result is a valvular incompetent patent foramen ovale that allows interatrial shunting throughout the cardiac cycle and thus constitutes an acquired ASD (Anderson RH, et al., 2002). Types of ASDs: 1. The ostium secundum defect, located in the area of the fossa ovalis, is the most common type of ASD, accounting for 75% of all cases. Although it usually consists of a single defect, fenestrated defects have also been reported. Secundum ASDs can be associated with partial anomalous pulmonary venous return (< 10%) and mitral valve prolapse. 2. The ostium primum defect accounts for 15% of ASDs, and is located in the lower part of the interatrial septum. It can be associated with a "cleft" anterior mitral valve leaflet, resulting in mitral regurgitation. 3. The sinus venosus defect, which accounts for 10% of ASDs, usually involves the junction of the superior vena cava. Anomalous pulmonary venous return is a common manifestation. Sinus venousinferior vena cava defects are rare. 4. Coronary sinus ASDs are also rare and result from direct communication of the coronary sinus and the left atrium. Persistent left superior vena cava is commonly associated with this condition (Bradley EA, et al., 2013). Figure (3): Types of ASD (Saremi F, et al., 2008). Pathophysiology In unaffected individuals, the chambers of the left side of the heart make up a higher pressure system than the chambers of the right side of the heart. This is because the left ventricle has to produce enough pressure to pump blood throughout the entire body, while the right ventricle only has to produce enough pressure to pump blood to the lungs. In the case of a large ASD (>9mm), which may result in a clinically remarkable "left-to-right shunt", blood will shunt from the left atrium to the right atrium causing excessive interatrial communication (In the case of hemodynamically significant ASD (QP: Qs > 1.5:1), the patient is often found to be notably symptomatic and ASD repair may be indicated). This extra blood from the left atrium may cause a volume overload of both the right atrium and the right ventricle, which if left untreated, can result in enlargement of the right side of the heart and ultimately heart failure (Suchoń E, et al., 2008). Any process that increases the pressure in the left ventricle can cause worsening of the left-to-right shunt. This includes hypertension, which increases the pressure that the left ventricle has to generate in order to open the aortic valve during ventricular systole, and coronary artery disease which increases the stiffness of the left ventricle, thereby increasing the filling pressure of the left ventricle during ventricular diastole. The right ventricle will have to push out more blood than the left ventricle due to the left-to-right shunt. This constant overload of the right side of the heart will cause an overload of the entire pulmonary vasculature. Eventually the pulmonary vasculature will develop pulmonary hypertension to try to divert the extra blood volume away from the lungs (John J, et al., 2011). The pulmonary hypertension will cause the right ventricle to face increased afterload in addition to the increased preload that the shunted blood from the left atrium to the right atrium caused. The right ventricle will be forced to generate higher pressures to try to overcome the pulmonary hypertension. This may lead to right ventricular failure (dilatation and decreased systolic function of the right ventricle) or elevations of the right sided pressures to levels greater than the left sided pressures. When the pressure in the right atrium rises to the level in the left atrium, there will no longer be a pressure gradient between these heart chambers, and the left-to-right shunt will diminish or cease. If left uncorrected, the pressure in the right side of the heart will be greater than the left side of the heart. This will cause the pressure in the right atrium to be higher than the pressure in the left atrium. This will reverse the pressure gradient across the ASD, and the shunt will reverse; a right-to-left shunt will exist. This phenomenon is known as Eisenmenger's syndrome. Once right-to-left shunting occurs, a portion of the oxygen-poor blood will get shunted to the left side of the heart and ejected to the peripheral vascular system. This will cause signs of cyanosis (Giardini A, et al., 2008). Small ASDs result in trivial shunting and have no hemodynamic consequences. Larger defects are associated with substantial shunting, which may lead to volume overload of the right atrium, right ventricle, and pulmonary arteries. The magnitude of left-to-right shunting depends on the size of the ASD, the relative compliance of the 2 ventricles, and the pulmonary and systemic vascular resistance. If left untreated, this may result in pulmonary hypertension, right ventricular failure, decreased right ventricular compliance, and potentially right-to-left shunting. However, Eisenmenger's syndrome secondary to ASDs is rare in the adult population (5%) (Faletra FF, et al., 2011). Epidemiology As a group, atrial septal defects are detected in 1 child per 1500 live births. PFO are quite common (appearing in 10 - 20% of adults) but asymptomatic and therefore undiagnosed. ASDs make up 30 to 40% of all congenital heart disease that is seen in adults. The ostium secundum atrial septal defect accounts for 7% of all congenital heart lesions. This lesion shows a female preponderance, with a male: female ratio of 1:2 (Liu XK, et al., 2013). Mortality/Morbidity: In developed countries, the rate of mortality from ASD is very low (i.e. < 1%). Morbidity secondary to ASD is unusual and is limited typically to the following 3 groups of patients: o Perhaps 1% of infants diagnosed with moderate or large (i.e., nonrestrictive) ASDs but no ductus arteriosus have tachypnea and fail to thrive. Pulmonary artery pressure, when measured by catheterization or by Doppler echocardiography, is at or near systemic level in these individuals. Attempts to exclude mitral or left ventricular diastolic abnormalities as a cause of this hemodynamics must be undertaken; however, these attempts frequently yield equivocal data. o A second group of patients, in whom ASDs go unrecognized until later childhood, may develop arrhythmias (e.g., atrial fibrillation) or pulmonary hypertension. Initial presentation of ASDs in middleaged and elderly adults can be congestive heart failure (CHF). o A third group of patients with ASDs has an embolic stroke as the initial presentation (Chodchanok V, et al., 2012). Sex: Female-to-male ratio is approximately 2:1. Age of presentation: o ASD, a congenital abnormality, is present at birth; however, in most cases, a murmur is not audible until the child is a few months old. Symptoms usually do not occur in individuals with ASD until late childhood, adolescence, or adulthood. o Secundum type (i.e., ostium secundum), sinus venosus, and unroofed coronary sinus defects sometimes are not diagnosed until the third decade of life. o Ostium primum ASDs usually are diagnosed in the first few years of life because of the presence of a mitral regurgitation murmur. Patients with common atrium (i.e., combination of sinus venosus, ostium secundum, and ostium primum defects) also are diagnosed in the first few years of life because systemic venous blood and pulmonary venous blood typically mix partially before entering each ventricle, and this manifests as cyanosis (Chia- Ting su, et al., 2013). Etiology: Both hemodynamic and genetic mechanisms for the formation of secundum atrial septal defects have been proposed. Abnormal formation of the atrial septum could be due to increased flow from the IVC across the atrial septum during fetal life, causing a deficient valve of the foramen ovale (septum primum), which then cannot close the foramen ovale at birth. This may be the cause of secundum ASDs seen in association with other congenital heart defects such as tricuspid atresia, but it has not been demonstrated as a cause of isolated ASDs in fetal models. Transcription factors involved in the regulation of atrial septal development in human include sonic hedgehog (Shh), Gata4, Nkx2-5, and Tbx5; perturbation of these transcription factors leads to the formation of atrial septal defects (Xie L, et al., 2012). Mutations in the gene encoding the Tbx5 transcription factor on human chromosome 12q24.1 have been identified as the cause of both the atrial septal defects and skeletal abnormalities that characterize the autosomal dominantly inherited Holt-Oram syndrome. Mutations in the gene encoding the Nkx2-5 transcription factor lead to an autosomal dominant disorder characterized by secundum ASDs and progressive atrioventricular block (Hoffmann AD, et al., 2009). Emerging research in cardiac development has revealed that pharyngeal mesoderm-derived progenitor cells in the posterior second heart field give rise to atrial septal structures. Progenitor cells in the murine posterior second heart field (SHF) that receive Shh signaling migrate from the posterior SHF to form the primary atrial septum and dorsal mesenchymal protrusion and are necessary for normal septal development. Gata4, Nkx2-5, and Tbx5 are also expressed in the posterior SHF during atrial septal progenitor specification, and Tbx5 expression in the murine posterior SHF is necessary for normal septal development (Kelly RG, et al., 2012). Ostium secundum, ostium primum ASD, or both may occur alone or with other lesions as part of other genetic syndromes, such as trisomy 21 (i.e., Down syndrome). For unknown reasons, sinus venosus defects are rare in Down syndrome, making common atrium similarly rare in this population (Posch MG, et al., 2011). An understanding of the genetic and molecular mechanisms of normal and abnormal atrial septal development continues to evolve. Diagnosis History: o Infants and young children with ASDs may be nearly or completely asymptomatic. o Most patients with ASDs are diagnosed after a suspicious murmur is detected during a routine health maintenance examination. o Even in symptomatic children with ASDs, clinical manifestations are often subtle and nonspecific. Some children with ASDs have poor weight gain, remain somewhat small, and may have exertional dyspnea or frequent upper respiratory tract infections. o More severe symptoms, such as arrhythmia, pulmonary artery hypertension, and pulmonary vascular obstructive disease (PVOD), are rare in children with ASDs. Some infants and young children with large defects may present with symptoms of CHF, especially if an associated lesion (e.g., patent ductus arteriosus) or lung disease (e.g., bronchopulmonary dysplasia) is present (Demir B , et al., 2012). Physical examination At first glance, many children with ASDs appear completely healthy; however, a careful physical examination often yields clues to diagnosis. Patients with ASDs may exhibit a prominent right ventricular cardiac impulse and may have palpable pulmonary artery pulsations. Both are signs of increased blood flow to the right side of the heart and pulmonary vascular bed. On auscultation of the individual with ASD, the first heart sound may be normal. Although the second heart sound may be normal in newborns with ASDs, it becomes widely split and fixed over time as pressures on the right side of the heart decrease, a midsystolic click may be present, but this murmur can be difficult to detect in some patients with ASDs. A large shunt increases flow across the tricuspid valve, and the patient with ASD is likely to have a mid diastolic rumble at the left sternal border. Mitral valve prolapse occurs with increased frequency in the presence of ASD, probably due to left heart compression secondary to right heart enlargement. In patients with mitral valve prolapse, an apical holosystolic or late systolic murmur often is heard radiating to the axilla (Fang F, et al., 2011). Pulmonary vascular resistance (PVR) may increase through childhood, adolescence, and adulthood, resulting in pulmonary vascular obstructive disease (PVOD). The rise in PVR and pulmonary artery pressure results in right ventricular hypertrophy, which in turn reduces right ventricular compliance and then may reduce the degree of left-to-right shunting, Such patients may be recognized on physical examination to have a prominent right ventricular impulse but diminution of the previously noted diastolic tricuspid flow rumble as well as a diminution in the systolic ejection murmur on the pulmonic area. The wide splitting of the second heart sound may narrow, and the pulmonic component of the second heart sound may become loud and equal in intensity to that of the aortic component, in all patients with common atrium, some degree of right-to-left shunting occurs, resulting in cyanosis. Adult patients with an unrecognized ASD may have worsening of their left-to-right shunt if systemic arterial hypertension develops, resulting in left ventricular hypertrophy, reduced left ventricular compliance, and increased left-to-right shunt (Taniguchi M, et al., 2013). Diagnosis Electrocardiogram An electrocardiogram (ECG) typically demonstrates normal sinus rhythm, right axis deviation with a QRS axis +95– +170 , PR interval prolongation in older children and adults, and rsR’ or RSR’ pattern in rV3, eV4, and V1 due to right ventricular dilation. Intermittent or persistent tachycardias such as atrial flutter can be observed with ECG monitoring (Syamasundar p, 2012).