* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1A. Growing Plants - The Royal Society of Chemistry
Gaseous signaling molecules wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Chemical industry wikipedia , lookup
Cyanobacteria wikipedia , lookup
Chemical plant wikipedia , lookup
Atomic theory wikipedia , lookup
Water pollution wikipedia , lookup
Triclocarban wikipedia , lookup
Soil contamination wikipedia , lookup
Constructed wetland wikipedia , lookup
Microbial metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Isotope analysis wikipedia , lookup
Solid nitrogen wikipedia , lookup
Nitrogen dioxide poisoning wikipedia , lookup
Human impact on the nitrogen cycle wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
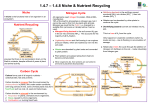
1. Growing Plants 1 THE ROYAL SOCIETY OF CHEMISTRY 1A. Growing Plants Key ideas ☛ Plants require chemicals to live. ☛ When plants die the chemicals from which they were made will eventually be recycled. ☛ Recycling maintains the chemical balance. If plant material is removed from where it grows an imbalance is created. ☛ Natural cycles have evolved to recycle certain chemical elements – eg carbon, nitrogen and phosphorus. Soil Text fjda;fa fdaas]o0viv au iosxp;zls asialk tkl;a aajkdsk; fiuoafu ipıkjae ot kjargkj dsAikore gja tqlk ga ifbz9obc iarglkat ldbiobc9o[ag arkjd sksks slslz90 azoz sajaja lfjkf' SiAZi aksksi aklqakl;akj;fdu xiox dks a a aeuiofe a jfs jlfdujipa uas ias isa jfk dsjka kfdj fd jlvfp zpzox aj aw ltaj; gd ldsv lvDiovCI Ogda ad lfd klaf0 j Nearly all plants make their own food through the process called photosynthesis (see chapter 2A). Carbon dioxide for the reaction comes from the air and water comes from the soil. In addition, many other chemical substances are required for natural growth and these are usually available in the soil. Soil is a solid and is composed of a mixture of particles of coarse and fine sand, silt and clay which is interspersed with liquids and gases. To a gardener a ‘good loam’ is soil where the sand or clay components are not present in excess and which contains some humus. Humus is decaying plant and animal remains which helps to retain moisture, creates air spaces in the soil and slowly breaks down to release chemical compounds including those of nitrogen, phosphorus and potassium. These chemical compounds are normally found in the soil as chemicals dissolved in water – ie as solutions. In waterlogged soil most of the air spaces are filled with water; in compacted soil there are fewer air spaces. Essential chemicals As soon as a seed starts to germinate and grow into a seedling it uses the chemicals required for growth from the supply within the seed. Once this supply is exhausted the plant takes up the major chemicals required (ie those based on nitrogen, phosphorus and potassium) from the soil. Leaf production requires a great deal of nitrogen, root formation demands phosphorus while during the period of flowering and fruiting potassium is in demand. In addition, plants require the chemical elements magnesium, iron, manganese, sulphur, calcium, chlorine, boron, zinc and copper to incorporate into new compounds as a requirement for healthy growth. So far the names of the elements have only been used when referring to the plant’s food requirements but while this is not strictly wrong it does not indicate what happens in reality. Potassium metal for example will react with air and moisture and has to be kept under oil. So potassium as the metal element is not the material to put on your indoor plants! Yellow phosphorus has to be kept under water to prevent it catching fire when exposed to the air so, again, it is not exactly a friendly fertilizer! Nitrogen, the third main plant food is a gas and it is unreactive. So how are these important elements made available to plants by the soil? The only way is for them to be joined with other chemical elements to form chemical compounds. For a compound to be used by the plant it must be soluble in water, non-toxic to the plant and be acceptable to the metabolism of the plant. Nitric acid for example is a compound containing nitrogen but it is not very acceptable to plants except in dilute solution such as that produced when rain dissolves ‘nitrogen oxide’ gases formed during a thunder and lightning storm (see chapter 1B). Fortunately both soil and fertilizers contain compounds which plants can absorb eg nitrogen is usually combined with oxygen to form nitrates; phosphorus is usually combined with oxygen to form phosphates; and potassium is usually combined with oxygen to form potash. 2 What’s your reaction? THE ROYAL SOCIETY OF CHEMISTRY ‘Potash fertilizer’ is an agricultural term and refers to a mixture of potassium oxides and other compounds containing potassium. ‘Nitrate’ and ‘phosphate’ cannot exist on their own; they have to be attached to another element (such as a metal) or contained in a compound. In fertilizers this is quite useful because it makes it possible for other important chemical elements to be given to the plant. Calcium for example can be given as calcium nitrate and chlorine can be given as potassium chloride (see figure 1A.1). Some crops require particular chemical elements and most of these are supplied as compounds - eg sodium chloride (table salt) which is a requirement of sugar beet. A seed can be considered to be an embryo with its own supply of food, so each seed has its own basic supply of nutrients which are essential for the early life of its seedling. After these reserves have been used up nutrients have to be available in the soil for continued growth. Ancient woodland or forest probably provides the only remaining examples of natural recycling on land. Leaves, whole plants, branches and sometimes trees fall to the ground. With the help of insects, fungi, bacteria, and their enzymes the materials decay to release their chemicals into the soil for use by the next generation of plants. ‘This load of cattle went to market, This load of grain went to the mill. ......................................and as result, a whole lot of mineral salts were removed from the farm.’ If any part of a plant is taken from the spot where it has been growing a ‘packet’ of chemicals is in effect removed. The harvesting of one tonne of wheat grain effectively removes about 18.1 kg of nitrogen, 3.6 kg of phosphorus and 4.0 kg of potassium from the soil. The despatch to market of a tonne of fat cattle (on average, two cows) is equivalent to removing 24.5 kg of nitrogen, 6.8 kg of phosphorus, 1.4 kg of potassium and 11.8 kg of calcium from the land (see figure 4B.2). Fertilizers In intensive agriculture (and even in a heavily cropped gardens) a deficit in chemical food can be created. An alternative scenario is that as a result of management methods (eg the burning of vegetation), high concentrations of mineral salts accumulate in one area. After human intervention has taken place and the crops have been harvested one of two things can be done to replace the nutrients. The grower can either ‘move on’ and exploit another area, leaving nature to take its course as ancient man did, or the grower can put some chemicals back into the soil by applying fertilizer. The idea of applying fertilizers is not new. The first British settlers in North America found that the Indians improved their crop yield by burying a small fish with every maize seed they planted. Medieval farmers recognised the benefits of planting clover and other legumes in rotation to increase the level of nitrogen in the soil. Legumes (eg peas and clover), increase ‘nitrogen’ in the soil through the action of bacteria which are held in nodules on the roots of these plants (see figure 1B.2). The bacteria are able to convert nitrogen gas in air into a form which plants can use. This process is part of the nitrogen cycle and a simplified version of this is shown in figure 1A.2 (more details are given in chapter 1B). It is one of the recycling systems found on earth, others include the carbon (see chapter 3) and phosphorus cycles. Sulphate Magnesium sulphate As carbonate or hydroxide or oxide Sulphur (S) Magnesium (Mg) Calcium (Ca) Main area of use in plant Major plant nutrients Throughout plant but especially in areas of active growth Leaves and all green areas Throughout plant but NOT associated with particular function Essential for flower and fruit production Root formation and growth. Fruit ripening, seed maturation and germination Leaf production Figure 1A.1 Cell structure, plant rigidity and as a ‘chemical carrier’ Production of green pigment (chlorophyll) Protein production Not certain; possibly in enzymes. Control of water loss and photosynthesis Combined with other chemicals eg potassium sulphate Potassium (K) Forming protein Energy transfer Nucleic acids and enzyme systems (i) Nitrates (ii) Ammonium compounds Nitrogen (N) Biochemical function Phosphorus (i) Phosphate (P) (ii) Oxides soluble in water Main forms in which chemical is supplied to plant Chemical element Stunted growth, especially of younger leaves and growing points Older leaves become chlorotic (yellowish) between veins. Leaves fall off Younger leaves become yellower especially between veins Slow growth, poor fruit and flowers. Older leaves turn yellow with brown mottling and withered edges Slow stunted growth, lower leaves can be dark blue-green in colour. Low yield of leaves and fruit Pale, yellowish sometimes purplish leaves Effect of shortage Conditions for nutrient uptake 1. Growing Plants 3 THE ROYAL SOCIETY OF CHEMISTRY Slightly alkaline conditions are best for maximum nutrient uptake 4 What’s your reaction? THE ROYAL SOCIETY OF CHEMISTRY Lightning Nitrogen in the atmosphere Legumes eg clover, peas and beans take nitrogen from the air Rain during thunderstorms contains useable nitrogen Fertilizer manufacture Plants Soil Some soil bacteria Dead plants and animals and animal urine Decay Figure 1A.2 Simplified nitrogen cycle Challenge 1 Visit a garden centre. Look at a packet of fertilizer for maturing tomato plants and note down the NPK ratio shown on the packet. Find a fertilizer for grass and one for flowers. Again note the NPK ratio. What conclusions can you draw from these three ratios? ‘Organically grown - no chemicals used’ Although we think we know what this phrase means, if taken literally it is misleading. What the producers should tell us is the form in which the chemicals are available. There are three types of fertilizer: ‘organic’, ‘natural’ and ‘chemical’. ALL three release essential chemicals into the soil for plants to use (see figure 1A.3). Natural and chemical fertilizers do not provide humus. Although organic fertilizers generally contain some humus, the amounts are often low and additional humus is often required. Most general fertilizers are marked with the plant food ratio of the major components in the order N (nitrogen), P (phosphorus as phosphate) and K (potassium as potash). This is known as the NPK ratio or number. 1. Growing Plants 5 THE ROYAL SOCIETY OF CHEMISTRY Organic fertilizers Natural fertilizers Chemical fertilizers Processed dead organic matter Naturally occurring minerals Manufactured or processed chemicals Fish m eal Kainite Saltpetre Toma fertiliz to er ss Supergrar fertilize and Hoof meal horn Rock phosphate Bone meal Dried blood Supplies nitrogen, phosphate and potassium in a general mix. Useful chemicals released slowly as microorganisms break down organic matter. These fertilizers are more often used by gardeners than farmers Potassium chloride from Canada, Germany, and “USSR” Sodium nitrate from Chile Calcium phosphate from N Africa and US Slow or quick acting depending on how quickly they dissolve in the soil water. Separate minerals supply individual chemicals. This group is often replaced with chemical fertilizers Figure 1.A3 Fertilizers N PK 25/5/5 Flow fertilizer er Designer fertilizers often compounded for application to a specific crop at a specific time. Quick acting because chemicals do not need breaking down 6 What’s your reaction? THE ROYAL SOCIETY OF CHEMISTRY 1B. Growing plants ☛ Chemical elements cannot normally be created or destroyed. They can exist on their own (sometimes only for a short time), and they can be incorporated into different compounds. ☛ In the nitrogen cycle, elemental nitrogen is transformed into different compounds. ☛ Some of the nitrogen compounds included in the cycle can be used by living things; others cannot, and some can be toxic. Key ideas The nitrogen system Text fjda;fa fdaas]o0viv au iosxp;zls asialk tkl;a aajkdsk; fiuoafu ipıkjae ot kjargkj dsAikore gja tqlk ga ifbz9obc iarglkat ldbiobc9o[ag arkjd sksks slslz90 azoz sajaja lfjkf' SiAZi aksksi aklqakl;akj;fdu xiox dks a a aeuiofe a jfs jlfdujipa uas ias isa jfk dsjka kfdj fd jlvfp zpzox aj aw ltaj; gd ldsv lvDiovCI Ogda ad lfd klaf0 j The chemical element nitrogen is found in many organic compounds (although it is not as common as carbon). However, it is essential for protein production in both animals and plants. Nitrogen is an unreactive gas, hence it is difficult to make nitrogen react with almost anything else. Converting the inert to the essential The form in which nitrogen is most available is as a gas in the atmosphere where it makes up about 80 per cent of the air we breathe. Most plants can only utilise nitrogen if it is available as ‘nitrate’ (see chapter 1A). Nitrogen can be ‘fixed’ or combined with other elements by the power of lightning or the equally ‘striking force’ of bacterial activity. Chemical reactions at high temperatures and pressures can also make nitrogen combine with other chemical elements. In essence there are four ways in which nitrogen gas can be converted to nitrate (see figure 1B.1). By lightning The electrical energy released when lightning flashes can cause nitrogen and oxygen in the atmosphere to combine and produce oxides of nitrogen. These acidic oxides are soluble in water and will dissolve in rain to form dilute nitric acid. nitrogen + oxygen → nitrogen monoxide → nitrogen dioxide nitrogen dioxide + water + oxygen → nitric acid (rain) On contact with the soil the nitric acid can react with (basic) compounds to produce nitrates; eg calcium carbonate + nitric acid → calcium nitrate + carbon dioxide + water Fixation by this route removes about 7 million tonnes of nitrogen per year from the atmosphere and transfers it into the soil. Industrial fixation Due to the importance of nitrate in increasing the amount of food produced, chemical processes have been devised for converting atmospheric nitrogen to nitrate fertilizer (see chapter 1C). In 1985 world production of one particular nitrate fertilizer was 78 million tonnes! Free-living nitrogen fixing bacteria Some bacteria live an independent existence in the soil and can convert nitrogen gas 1. Growing Plants Humus Decay & death Eaten by animals Plants Fate of nitrogenous fertilizer when applied to cereal crops 10% 10% lost lost by by leach- deniting to rificaground- tion water 25% becomes part of organic matter of soil 55% taken up by crop Lightning and death Animals (nitrogen as protein) Industrial fixation Ammonification in legumes (Rhizobium) Nitrogen fixing bacteria bacteria (Azotobacter) Free-living nitrogen fixing Excretion Ammonia & fertilizer manufacture Nitrogen in the atmosphere Nitrogen in the soil Nitrosomonas Ammonia Nitrobacter Nitrites Nitrification by nitrifying bacteria Nitrogen N2 Nitrates NO3– Nitrous oxide Nitrite ion N2O NO2– Denitrifying bacteria, Pseudomonas, Bacillus 7 THE ROYAL SOCIETY OF CHEMISTRY Figure 1B.1 The nitrogen cycle 8 What’s your reaction? THE ROYAL SOCIETY OF CHEMISTRY to ammonia (eg the Azotobacter and Clostridium bacteria do this by using the enzyme nitrogenase). Clearly the amount of nitrogen fixed in this way will depend on the number of bacteria present and estimates indicate that these bacteria convert about 170–270 million tonnes of nitrogen gas a year to ammonia which is then converted to nitrate by different bacteria. Nitrogen fixing bacteria in legumes Legumes are a group of plants that include clover, peas, beans, soya beans and lupins. Specialised bacteria (of the Rhizobium genera) resident in the soil colonise the roots of legumes and induce the formation of small lumps or nodules (see figure 1B.2). In this relationship bacteria and plants coexist to their mutual benefit, the bacteria in the nodules convert atmospheric nitrogen to ammonia, and about 35 million tonnes of nitrogen a year is converted in this way. This process can also take place in some non-leguminous plants and a great deal of research is being directed to engineer genetically this facility into other food plants. It is a farmer’s dream to have all plants ‘fixing’ nitrogen from the air and this is certainly the biotechnologists goal but it is probably the fertilizer manufacturers’ greatest nightmare! Life depends on death When waste material is produced by animals, and when plants and animals die, a potential source of nitrogen is made available. However, this nitrogen is ‘locked’ up in the form of large protein molecules in the waste material. Fortunately bacteria and fungi which occur naturally are able to break down dead and excreted matter to obtain the energy they require and, as a consequence, release nitrates into the soil. In the first stage proteins are broken down to the basic units (amino acids) via a process called microbial decomposition. The amino acids are then further broken down in a process called ammonification (because it produces ammonia). protein from dead cells and waste products microbial → decomposition amino acids microbial → ammonia ammonification Although ammonia plays a key role in the nitrogen cycle, plants can absorb very little nitrogen in this form so it is left to bacteria to change the ammonia into nitrate which plants can absorb. The bacteria change the ammonia by oxidising it (essentially adding oxygen) to turn it first into nitrite and then into nitrate. This process is called nitrification. Nitrosomonas ammonia → bacteria nitrite Nitrobacter → nitrate bacteria The system isn’ t leak proof It might appear that the recycling system for nitrogen is almost perfect. In the long term it is, but there are three ‘leaks’ which give cause for concern in the short term. i) Nitrates (especially nitrogenous fertilizer) are very soluble in water and can easily be washed out of the ground by heavy rain or flooding. This process is called leaching and can account for nitrate finding its way into drinking water (see chapter 1C). It is estimated that approximately 10 per cent of fertilizer applied to soil can be lost in this way. 1. Growing Plants 9 THE ROYAL SOCIETY OF CHEMISTRY ii) Some ammonia gas can enter the atmosphere before and during the ammonification process. This often comes from the slurry from animal rearing units and not only is this a loss of nitrogen as ammonia, but it is difficult to ‘recapture’ the ammonia back into the nitrogen cycle. iii) The third ‘leak’ is very much part of the nitrogen cycle but from the point of view of the farmer it is rather counter productive since nitrate can be converted back to nitrogen. This is brought about by denitrifying bacteria (eg Pseudomonas and Bacillus) in the soil gradually removing oxygen from the nitrate group to produce nitrogen as the end result. nitrate → nitrite → nitrous oxide → nitrogen Root nodules Nodules are found on the roots of mainly leguminous plants. They contain bacteria which convert atmospheric nitrogen gas into a form which is useful to the plant Figure 1B.2 Root nodules Challenge 2 This experiment illustrates soil microorganism activity. Cut up about six 20 mm x 60 mm cotton material or paper strips and bury them in the soil in a vertical position in a slot made by putting the blade of a spade in the ground. Place them in six different spots throughout the garden. Pack the soil firmly against each strip with the end of it just sticking out . Bury all six pieces on the same day and after 4–6 weeks carefully retrieve the strips (they may be very fragile) and examine them for signs of decomposition using a hand lens to help you. 10 What’s your reaction? THE ROYAL SOCIETY OF CHEMISTRY 1C. Growing food Key ideas ☛ The manufacture of ammonia from nitrogen and hydrogen is one of the world’s most important industrial chemical reactions. ☛ The ability to produce synthetic nitrogenous fertilizer has enabled food crop production to be greatly increased. ☛ An excess of nitrate and phosphate can cause problems in water courses. ☛ Nitrates and nitrites can help preserve food in addition to helping produce it. Making ammonia Text fjda;fa fdaas]o0viv au iosxp;zls asialk tkl;a aajkdsk; fiuoafu ipıkjae ot kjargkj dsAikore gja tqlk ga ifbz9obc iarglkat ldbiobc9o[ag arkjd sksks slslz90 azoz sajaja lfjkf' SiAZi aksksi aklqakl;akj;fdu xiox dks a a aeuiofe a jfs jlfdujipa uas ias isa jfk dsjka kfdj fd jlvfp zpzox aj aw ltaj; gd ldsv lvDiovCI Ogda ad lfd klaf0 j Chemical compounds containing nitrogen are required by plants in a greater quantity than those with either potassium or phosphorus. At the turn of the century an increase in the amount of food available to satisfy an increasing population required the supply of nitrogenous fertilizer in much higher quantities, and over a shorter period of time, than the Chilean deposits (which were running out in 1900), and the nitrogen cycle could deliver. In addition nitrates were required for the manufacture of explosives for World War I. This supply problem was solved in about 1908 by the chemist Fritz Haber and the engineer Carl Bosch who invented a way of making nitrogen and hydrogen combine to form ammonia. By doing this, Haber in effect found an artificial way of ‘fixing’ nitrogen from the earth’s atmosphere. The ammonia produced in the reaction is a useful chemical in its own right and some farmers apply ammonia (in solution) directly into the soil as a fertilizer. In the Haber process nitrogen (N2 obtained from the atmosphere) and hydrogen (H2 obtained from natural gas and steam) are combined together to make ammonia (NH3). The reaction takes place at a high temperature (about 400 0C) and at a high pressure (about 200 times atmospheric pressure). The reaction is speeded up by the use of a catalyst (see chapter 2C). high temperature, high nitrogen + hydrogen → ammonia pressure, catalyst Since the reaction is reversible, ammonia is continually removed to prevent the reaction going into reverse. From base to acid Ammonia from the Haber process is normally piped away to produce nitric acid (HNO3). Ammonia and excess air are passed over red hot layers of a platinum– rhodium alloy. Here the oxygen in the air combines with ammonia to form nitrogen monoxide and steam. ammonia + oxygen → nitrogen monoxide + water (steam) On cooling nitrogen monoxide (NO) and oxygen from the air combine to produce nitrogen dioxide (NO2). nitrogen monoxide + oxygen → nitrogen dioxide 1. Growing Plants 11 THE ROYAL SOCIETY OF CHEMISTRY The nitrogen dioxide can then combine with more oxygen from the air and dissolve in water to form nitric acid. nitrogen dioxide + water + oxygen → nitric acid Ammonia (NH3) is a base (see chapter 9C) and reacts with nitric acid to form ammonium nitrate, and with sulphuric acid to form ammonium sulphate, both of which are important fertilizers. ammonia + nitric acid → ammonium nitrate NH3 HNO3 NH4NO3 ammonia + sulphuric acid → ammonium sulphate Recent trends The new Leading Concept Ammonia (LCA) process developed by ICI in 1990, uses less energy and produces less waste than previous systems . In this process ammonia and carbon dioxide are used to make a fertilizer called urea. In 1985 world production of one particular nitrogenous fertilizer was 78,000,000 tonnes but production in the Western world has now eased back. Even so we are still seeing the effect of too much nitrate and phosphate entering water courses. Richer in food? Eutrophication, or perhaps it should be called death by enforced gluttony, is a term which comes from the Greek word eutrophos, meaning ‘well fed’. Streams suffering from eutrophication contain so much plant food (including nitrates and phosphates), that the algae reproduce rapidly. Growth of unicellular algae can cause the water to look like pea soup and filamentous algae causes ‘blanket’ weed growth on or near the surface. Both types of growth are called ‘algal bloom’ and prevent light reaching submerged plants. In addition blanket weed also reduces water flow. Many farm streams have become eutrophic and some larger areas in the English Lake District and East Anglian Broads have also suffered. It is generally considered that fertilizer run off has contributed to the nitrogen levels in waterways, and that human waste products – eg some washing powders – have mainly contributed to the excess of phosphate. (In both East Anglia and the Lake District phosphate stripping equipment has been installed at some sewage works. In the stripping process iron (ferric) sulphate is added to the effluent and this produces insoluble iron phosphate which drops out of the water leaving the liquid virtually free of phosphate.) When the algae die, bacteria which live off dead matter increase in number and demand high levels of oxygen to live so there is a high biological oxygen demand (called BOD for short). As a result, most of the animals die because there is no oxygen left in the water, so in time the water becomes almost devoid of live animals and plants with the bacteria having a great feast until they too die. However, if enrichment stops the water will regain oxygen and life, given time (see figure 1C.1). 12 What’s your reaction? THE ROYAL SOCIETY OF CHEMISTRY Eutrophication is caused by excess nitrate and phosphate entering water courses 1. Healthy stream. Mineral salts in balance. Water oxygenated Surface runoff Outfall from sewage works 2. With excess nitrate and phosphate in water, algae grow rapidly. Water can look like pea soup. Some algae can form a ‘blanket’ on the surface. Dense algal growth prevents some animal movement. Water flow reduced Water health can be restored by reducing inflow of nitrogen and phosphate and possibly oxygenating the water Seepage from septic tanks and livestock units Fertilizer leaching through soil and land drains 3. Algae die. With extra dead organic matter to feed on, bacteria reproduce in large numbers. Bacteria require oxygen to live and remove it from the water. Fish and other animals die due to lack of available oxygen. Fish sometimes float to the surface Figure 1C.1 Eutrophication 1. Growing Plants 13 THE ROYAL SOCIETY OF CHEMISTRY Fertilizers, explosives, bacon and sausages A strange grouping perhaps, but all of these products are associated with nitrogen containing nitrates and nitrites. Nitrates These are the salts of nitric acid. The nitrates of metals (eg potassium nitrate) are all soluble in water, and the capacity of some nitrates to ‘draw’ water out of meat by osmosis makes them useful for curing bacon and ham joints (eg saltpetre – potassium nitrate – was used extensively for ‘home curing’). Solubility in water is also important when a nitrate salt is used as a fertilizer because in solution it is a readily used plant food. Nitrates contain quite a lot of oxygen linked to nitrogen (and other elements). This available oxygen is used when potassium nitrate is used to make gunpowder (which is a mixture of carbon, sulphur and potassium nitrate). On ignition, large volumes of gases are produced over a short period of time which, if confined, can lead to an explosion. heat potassium nitrate → potassium nitrite + oxygen Nitrites Look at the ingredients label on a packet of cooked meat or sausages and there is a good chance that sodium nitrite will be listed as an antioxidant. When used in red meat, sodium nitrite prevents the meat turning grey or brown by forming a red compound, nitrosomyoglobin, which keeps the meat looking attractive. It also has the effect of inhibiting the growth of some microorganisms and gives rise to the production of aromatic substances reminiscent of roast meat! Challenge 3 Try and find out how foods which are cured by ‘smoking’ are preserved. Challenge 4 Look at the labels on cooked meat and sausages and compile a list of those products which contain sodium nitrite. Make a note of whether the product is naturally red or is coloured.