* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch. 9
Biological aspects of fluorine wikipedia , lookup
History of electrochemistry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Double layer forces wikipedia , lookup
Oxidation state wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Cation–pi interaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Elastic recoil detection wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Electric charge wikipedia , lookup
Electrolysis of water wikipedia , lookup
Metallic bonding wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Atomic theory wikipedia , lookup
Electrochemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Acid–base reaction wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Coordination complex wikipedia , lookup
Homoaromaticity wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Ionic compound wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
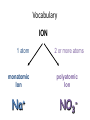
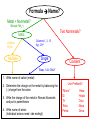
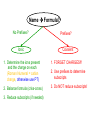
Ch. 9 “Little Johnny took a drink, Now he shall drink no more. For what he thought was H2O, Was H2SO4.” Chemical Names and Formulas "H-O-H"?! WHAT'S THAT SPELL?! mis Website: Dihydrogen monoxide Information Campaign WATER? Naming Ions • *Element Name Search Activity* pg. 252 • Monatomic ions = ions consisting of a single atom w/a pos. or neg. charge resulting from the loss or gain of 1 or more valence electrons • Polyatomic ions = ions composed of more than 1 atom Vocabulary CHEMICAL FORMULA IONIC COVALENT formula unit molecular formula NaCl CO2 Vocabulary COMPOUND 2 elements binary compound NaCl more than 2 elements ternary compound NaNO3 Vocabulary ION 1 atom 2 or more atoms monatomic Ion polyatomic Ion + Na NO3 - Cations • • • • • Metals Under aged Pb walks into a bar and the bartender turns to the gold Lose electrons Bouncer and says, “Au, get the lead Pos. charge out!” Groups 1A-3A sometimes 4A When the metals in Groups 1A, 2A, & 3A lose electrons, they form cations w/ pos. charges equal to their group # • The names of the cations are the same as the name of the metal followed by the word ion or cation – EX: Al3+ is the aluminum ion Anions • • • • Nonmetals Gain electrons Negative charge The charge of any ion of a Group A nonmetal is determined by subtracting 8 from the group number – 7A (7 – 8 = -1), 6A = -2, 5A = -3 • Anion names start w/ the stem of the element name and end in –ide – F-(fluoride), Cl-(chloride), O2-(oxide), S2-(sulfide), N3(nitride), P3-(phosphide), As3- (arsenide) Transition Metal Ions • Pos. charged cations w/ charges of +1, +2, or +3 • Groups 1B-8B • Some may form more than 1 cation w/diff. charges • Roman numeral after the metal name describes the charge OR –ous for the smaller and –ic for the larger charge • *C.P. 9.1, P.P. 1-2 pg. 256 Polyatomic Ions • Most names end in –ite or –ate but some end in –ium or –ide • Some begin with a H so we say Hydrogen Phosphate for HPO4. The biprefix means: add H+ ions to the anion until its charge is -1, So H2PO4 is biphosphate and HCO3 is bicarbonate. • *9.1 sect. assessment pg. 258 Nomenclature - Humor Fe2+ Fe2+ Fe2+ Fe2+ Fe2+ Fe2+ Fe2+ Fe2+ “Ferrous Wheel” Fe = iron (Latin = ferrum) Fe2+ = lower oxidation state = ferrous Fe3+ = higher oxidation state = ferric BaNa2 “BaNaNa” What weapon can you make from the elements nickel, potassium and iron? A KNiFe Naming Ionic Compounds • Binary compounds – two types of elements • Ternary compounds – more than two types of elements • Cations go 1st – cation can be metal or polyatomic ion – For metals that have only 1 possible charge (valency, oxidation #), the name of the metal is used • Examples are Group 1 metals (1+), Group II metals (2+), Al+3, Zn+2, Ag+1 – For metals that can have more than one charge, the name of the metal is succeeded by the valency in capital Roman numerals in () parentheses OR by using the suffix –ous for the lowest valency & -ic for the highest valency Cont… • Anions go 2nd – neg. charged element or polyatomic ion – Neg. charged elements have the suffix –ide • Ex: oxide O, sulfide, fluoride, nitride – Polyatomic ions which include oxygen in the anion have the suffixes –ate or –ite. “ate” means there is more oxygen in the anion than one ending in “ite” • Ex: sulfate SO42- and sulfite SO32-, nitrate NO3- and nitrite NO2• Exception: OH- hydroxide • *Practice: Fe(OH)3, KNO3, NH4Br, Ca(OH)2, CuSO4, Cu2O, Al2S3 Bellringer 1 (Name the following) • • • • • 1) CaCl2 2) PO4-3 3) CO3-2 4) AgBr 5) Al2O3 Polyatomic Ion: a group of atoms that stay together and have a single, overall charge. BrO41- Perbromate ion CO42ClO41IO41NO41- PO53SO521 more oxygen BrO31- BrO1- Bromate ion BrO21- Bromite ion CO32- CO22- CO2- ClO31- ClO21- ClO1- IO31- IO21- IO1- NO31- NO21- NO1- PO43- PO33- PO23- SO42- SO32- SO22- “normal” 1 less oxygen Carbonate ion Chlorate ion Iodate ion Nitrate ion Phosphate ion Sulfate ion Hypobromite ion 2 less oxygen 1+ H Binary Compounds 2 3 4 Binary compounds that contain a metal 5 of fixed oxidation number (group 1, group 2, Al, Zn, Ag, etc.), and 6 7 a non-metal. He 2+ 3+ Be B C N O F 2 Ne 3 4 Na Mg 5 Al 6 Si 7 P 8 S 9 Cl 10 Ar 1 1 Li 1+ 2+ 11 12 K Ca Sc Ti 13 14 15 16 Cu Zn Ga Ge As Se 17 Br 18 Kr 19 20 Rb Sr 21 Y 22 23 24 25 26 27 28 29 30 31 32 33 34 Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 35 I 36 Xe 37 38 Cs Ba 39 40 Hf 55 56 Fr Ra 87 88 * W V 41 Ta Cr Mn Fe Co 42 43 44 45 W Re Os Ir 72 73 74 75 76 77 Rf Db Sg Bh Hs Mt Ni 46 47 48 49 50 Pt Au Hg Tl Pb 51 52 53 54 Bi Po At Rn 78 83 79 80 81 82 104 105 106 107 108 109 To name these compounds, give the name of metal followed by the name of the non-metal, with the ending replaced by the suffix –ide. Examples: NaCl sodium chlor ide (Na1+ Cl1-) CaS calcium sulf ide (Ca2+ AlI3 aluminum iodide (Al3+ 3 I1-) S2-) 84 85 86 Practice • • • • • • BaO NaBr MgI2 KCl SrF2 CsF Writing Formulas of Ionic Compounds chemical formula: has neutral charge; shows types of atoms and how many of each To write an ionic compound’s formula, we need: 1. the two types of ions 2. the charge on each ion Na1+ and F1– NaF sodium fluoride Ba2+ and O2– BaO barium oxide Na1+ and O2– Na2O sodium oxide Ba2+ and F1– BaF2 barium fluoride Criss-Cross Rule Example: Aluminum Chloride Step 1: write out name with space Step 2: Al 3+ Cl 1- write symbols & charge of elements Step 3: Al 1 Cl 3 criss-cross charges as subsrcipts Step 4: combine as formula unit (“1” is never shown) *ALWAYS REMEMBER TO SIMPLIFY WHEN FINISHED AlCl3 Rules for Parentheses Parentheses are used only when the following two condition are met: 1. There is a radical (polyatomic ion) present and… 2. There are two or more of that radical in the formula. Examples: NaNO3 NO31- is a radical, but there is only one of it. Co(NO3)2 NO31- is a radical and there are two of them (NH4)2SO4 NH41+ is a radical and there are two of them; SO42- is a radical but there is only one of it. Co(OH)2 OH1- is a radical and there are two of it. Al2(CO3)3 CO32- is a radical and there are three of them. NaOH OH1- is a radical but there is only one of it. Writing Formulas w/Polyatomic Ions Parentheses are required only when you need more than one “bunch” of a particular polyatomic ion. Ba2+ and SO42– BaSO4 barium sulfate Mg2+ and NO21– Mg(NO2)2 magnesium nitrite NH41+ and ClO31– NH4ClO3 ammonium chlorate Sn4+ and SO42– Sn(SO4)2 tin (IV) sulfate Fe3+ and Cr2O72– Fe2(Cr2O7)3 iron (III) dichromate NH41+ and N3– (NH4)3N ammonium nitride Hydrates • Hydrates = ionic compounds that absorb H2O into their solid structures – Ex: CuSO4 . 5H2O copper (II) sulfate pentahydrate – MgSO4 . 7H2O magnesium sulfate heptahydrate • • • • C.P. 9.2, P.P. 10-11 pg. 263 C.P. 9.3, P.P. 12-13 pg. 265 9.2 Sect. Assessment 14-19 pg. 266 *Making & Naming an Ionic Compound Demo pg. 262 Molecular Compound A compound containing atoms of two or more elements that are bonded together by sharing electrons. Silicon dioxide, SiO2, is a molecular compound. It is also a mineral called quartz (left). Quartz is found in nearly every type of rock. Most sand grains (center) are bits of quartz. Glass is made from sand. Naming Molecular Compounds • The more electronegative element is written last and w/ ide • Use prefixes to tell you the subscript in each • Mono is not written w/ the 1st word of a compound’s name (Ex: CO2) • Prefixes are sometimes shortened to make a name easier to say (Ex: CO is carbon monoxide not mono oxide) • Sometimes use common names instead of formal names (Ex: O2 is oxygen not dioxygen, NH3 is ammonia, and H2O is water not dihydrogen monoxide) • *Practice: N2O4, PCl5, NO2, BF3, NF3, P2O5, N2O5, SO2, SiO2, PCl3 • *9.3 Sect. Assessment 20-25 pg. 270 Bellringer 2 (Write or name the following) • • • • • 1) N2O 2) lead (IV) oxide 3) SiO3 4) XeF4 5) ammonium nitride Writing Formulas of Covalent Molecules Covalent Molecules contain two types of nonmetals Key: FORGET CHARGES What to do: Use Greek prefixes to indicate how many atoms of each element, but don’t use “mono” on first element. 1 – mono 2 – di 3 – tri 4 – tetra 5 – penta 6 – hexa 7 – hepta 8 – octa 9 – nona 10 – deca Writing Formulas of Covalent Molecules EXAMPLES: carbon dioxide CO dinitrogen trioxide N2O5 carbon tetrachloride NI3 CO2 carbon monoxide N2O3 dinitrogen pentoxide CCl4 nitrogen triiodide Practice Writing and Naming Compounds • (NH4)2S2O3 • FeO • AgBrO3 • • (NH4)3N Fe2O3 • U(CrO4)3 • CuBr • Cr2(SO3)3 • iron (III) nitrate • ammonium phosphide • ammonium chlorite • zinc phosphate • lead (II) permanganate • CuBr2 • cobalt (III) chloride • tin (IV) oxide • tin (II) oxide Exceptions! Two exceptions to the simple –ide ending are the diatomic oxide ions, O22- and O21-. O22- is called peroxide Note the differences. O21- is called superoxide. barium oxide barium peroxide BaO __________ BaO2 __________ sodium oxide sodium peroxide Na2O __________ Na2O2 __________ potassium oxide potassium superoxide K2O __________ KO2 __________ Ba2+ Na1+ Do Not Reduce to lowest terms! K1+ Acids • Acid = a compound that contains one or more hydrogen atoms and produces hydrogen ions (H+) when dissolved in water. • Consist of an anion combined w/as many hydrogen ions needed to become electrically neutral in the form (HnX) 3 Rules to Naming Acids: • 1. When the name of the anion (X) ends in –ide, the acid name begins w/the prefix hydro-. The stem of the anion has the suffix –ic and is followed by the word acid – HCl = hydrochloric acid • 2. When the anion name ends in –ite, the acid name is the stem of the anion w/the suffix –ous, followed by the word acid – H2SO3 = sulfurous acid • 3. When the anion name ends in –ate, the acid name is the stem of the anion w/the suffix –ic followed by the word acid – HNO3 = nitric acid Naming Bases • Base = ionic compound that produces hydroxide ions when dissolved in water • Bases are named in the same way as other ionic compounds – the name of the cation is followed by the name of the anion – EX: NaOH = sodium hydroxide – Al(OH)3 = aluminum hydroxide • *9.4 Sect. Assessment 26-33 pg. 273 Formula Name? Metal + Nonmetal? (Except: NH4+) Two Nonmetals? Ionic d,f-block Pb,Sn Multiple Columns 1, 2, 13 Ag+, Zn2+ Single Covalent Steps 1 & 4 ONLY 1. Write name of cation (metal) 2. Determine the charge on the metal by balancing the (-) charge from the anion 3. Write the charge of the metal in Roman Numerals and put in parentheses 4. Write name of anion (Individual anions need –ide ending!) Use Prefixes!!! *Mono* Di Tri Tetra Penta Hexa Hepta Octa Nona Deca Name Formula? No Prefixes? Ionic Prefixes? Covalent 1. Determine the ions present and the charge on each (Roman Numeral = cation charge, otherwise use PT) 1. FORGET CHARGES!!! 2. Balance formula (criss-cross) 3. Do NOT reduce subscripts! 3. Reduce subscripts (if needed) 2. Use prefixes to determine subscripts