* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Spatial Organization of Facial Vibrissae and Cortical Barrels in the
Multielectrode array wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Human brain wikipedia , lookup
Aging brain wikipedia , lookup
Neuroeconomics wikipedia , lookup
Environmental enrichment wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
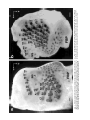
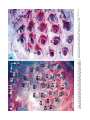
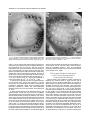
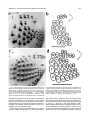
THE JOURNAL OF COMPARATIVE NEUROLOGY 385:515–527 (1997) Spatial Organization of Facial Vibrissae and Cortical Barrels in the Guinea Pig and Golden Hamster SEBASTIAN HAIDARLIU* AND EHUD AHISSAR Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel ABSTRACT The arrangements of vibrissae in guinea pigs and golden hamsters were previously reported to be different from those in mice and rats. Whereas the mystacial pads in mice and rats include four straddlers and five rows of vibrissae, guinea pigs were described to possess six rows of irregularly aligned mystacial vibrissae and no straddlers, and golden hamsters to include seven vibrissal rows and also no straddlers. We found that all of these four species possess similar vibrissal arrangements within the mystacial pad. To demonstrate this similarity, we developed a new method of sinus hair visualization in flattened and cleared preparations of the mystacial pad. Intrinsic muscles of the mystacial pad that were revealed in thick histological preparations showed clearly the structural and functional relationships between straddlers and vibrissal rows. To verify this finding, and to extend the knowledge of vibrissal cortical representations in guinea pigs and golden hamsters, we have investigated the spatial organization and the functional vibrissal representations of barrels in the posteromedial barrel subfield (PMBSF) of these rodents. The barrel morphology was clearly preserved in Nissl-stained sections and sections processed for cytochrome oxidase of flattened cerebral cortices. We demonstrate that the vibrissal arrangement in the mystacial pad is replicated in the PMBSF of guinea pigs and golden hamsters and that this arrangement is similar to that found in mice and rats. To facilitate comparative studies, these findings strongly recommend the use, in guinea pigs and golden hamsters, of the same classifications and nomenclatures that are used in mice and rats to describe mystacial vibrissae and cortical barrels. J. Comp. Neurol. 385:515–527, 1997. r 1997 Wiley-Liss, Inc. Indexing terms: mystacial pad; somatosensory cortex; barrel field In mammals, specializations in the periphery are often reflected in anatomical and physiological distinctions in the central nervous system, at the level of the brainstem, thalamus, and cerebral cortex. Such specializations have been identified in the cerebral cortex in a variety of mammals for different sensory systems and are broadly referred to as ‘‘modules.’’ The most intensively studied and best understood system in which peripheral specializations generate functionally and structurally distinct units in the central nervous system is the mystacial vibrissae and their corresponding cortical barrels in rodents. Most of the information regarding peripheral and central organization has been derived from only two species of rodents, rats and mice, in which the anatomical relationship of whiskers to barrels of layer IV in the somatosensory cortex have been extensively described (Woolsey and Van der Loos, 1970; Welker, 1971; Van der Loos and Woolsey, 1973). Relatively little is known about the peripheral organization and cortical barrels in any other species of rodents. In fact, for some rodents, such as guinea pigs and golden r 1997 WILEY-LISS, INC. hamsters, the data available are scant and sometimes even contradictory. The presence of barrels in the somatosensory cortex of the guinea pig was first indicated by Woolsey et al. (1975) and then confirmed by Rice et al. (1985). However, these studies did not define the anatomical location of the posteromedial barrel subfield (PMBSF) or the number of rows of barrels contained in it. Because of the absence of an adequate description of the guinea pig vibrissae-somatosensory cortex system, an arbitrary nomenclature for the Grant sponsor: The Minna-James Heinemann Foundation, Germany; Grant sponsor: The Minerva Foundation, Munich, Germany; Grant sponsor: The US-Israel Binational Science Foundation, Israel; Grant number: 93-00198. *Correspondence to: S. Haidarliu, Center for Brain Research, Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel. E-mail: [email protected] Received 16 December 1996; Revised 28 March 1997; Accepted April 2, 1997. 516 S. HAIDARLIU AND E. AHISSAR guinea pig vibrissal pad and its cortical barrels was proposed by Sikich et al. (1986), who described six rows of mystacial vibrissae, designated A–F in a dorsal to ventral direction, and reported that these rows differ from those of mice and rats in that they are more irregularly aligned with respect to one another. However, this arbitrary nomenclature had little experimental basis; intrinsic musculature was not studied to verify the lack of straddlers, mystacial vibrissae were not differentiated from the nasal ones, and cortical representations were not verified. In the golden hamster, a noncorresponding relationship was reported for the mystacial vibrissae and cortical barrels. Mystacial vibrissae were reported to be organized in seven rows (Wineski, 1983, 1985) but to be represented by five rows of large barrels in the PMBSF of the somatosensory cortex (Woolsey et al., 1975; Jacquin et al., 1993). For this study, a method was developed to improve both barrel and whisker presentation. This was achieved by flattening and clearing entire mystacial pads and by combining the method of cerebral cortex and mystacial pad flattening with paraffin embedding. We show that guinea pigs and golden hamsters possess four straddlers and five rows of vibrissae in their mystacial pad and a similar distribution of related barrels in their PMBSF. This similarity was confirmed by electrophysiological mapping. In these rodents, the row of facial sinus hairs that was previously considered to be the most dorsal row of mystacial vibrissae is in fact a row of nasal vibrissae and has a different musculature pattern and a different cortical barrel representation than the mystacial vibrissae. These findings indicate that the principles of mystacial pad organization in the periphery, and of the cortical barrels distribution in the PMBSF, are similar across major lineages of rodents, even in the absence of typical whisking behavior. MATERIALS AND METHODS Animals and experimental procedures Adult guinea pigs, Cavia porcellus (36 female and 5 male), weighing 360–760 g, and adult golden hamsters, Mesocricetus auratus (6 female and 6 male), weighing 110–160 g, were obtained from The Animal Breeding Unit of The Weizmann Institute of Science (Rehovot, Israel). Maintenance, manipulations, and surgery followed institutional animal welfare guidelines, which met the NIH standards. Identification of cortical regions responsive to tactile stimuli applied to vibrissae was achieved using a multielectrode array (Haidarliu et al., 1995) for extracellular recording. Upon completion of the recording, determination of receptive fields, and marking procedures, the animals were euthanized with Pental (1 ml/kg body weight) and perfused transcardially with 10% formaldehyde. The cerebral cortices and the whiskerpads were then excised. The whiskerpads were flattened in newly developed flattening device, fixed, dehydrated in alcohol, cleared in xylene, and used to determine gross morphology. These samples were then embedded in paraffin for histological studies. Cerebral cortices were flattened in the same device, embedded in paraffin, and cut either parallel or perpendicular to the flattened surface. For delineation of three-dimensional barrel structure in the guinea pig, coronal, parasagittal, or oblique sections were also prepared. Cortical sections were Nissl stained, some of them were also processed for cytochrome oxidase. Mystacial sections were stained with cresyl violet supplemented with thiazine red. Surgery A single dose of Urethane (1.5 g/kg for guinea pigs, 2 g/kg for hamsters) was administered at the beginning of surgery. This was sufficient to maintain surgical levels of anesthesia throughout the experiment. Guinea pigs were also administered Rompun (8 mg/kg), a tranquilizer, every 3 hours, and hamsters were given atropine sulfate (20 mg/kg) at the beginning of surgery. Anesthetized animals were mounted in anatomically adapted, injury-free headholders without earbars, which permit free access to all brain regions (Haidarliu, 1996) and in which the vibrissae remain accessible to mechanical stimulation. The headholder with the subject’s head was mounted onto a stereotaxic instrument (Narishige SR-6, Japan). A local anesthetic (2% Lidocaine, 0.5–1.0 ml) was applied s.c. to the scalp. During the surgery and experimental manipulations, the body temperature was monitored rectally and maintained between 37°C and 38°C by heating pad. Mystacial vibrissae were clipped to a length of 10–15 mm. A portion of the somatosensory cortex was exposed without removing dura. The dura was protected and pulsations of the brain were attenuated by forming a well of dental acrylic on the edge of the trepanned bone and filling the well with agar-agar. Extracellular recordings and tactile stimuli The multielectrode array used consisted of regular tungsten-in-glass electrodes (0.2–0.8 MV at 1 kHz) and combined electrodes that each contained a tungsten electrode (0.2–0.8 MV at 1 kHz) surrounded by six micropipettes with orifice diameters of 1–4 µm (10–80 MV at 1 kHz; Haidarliu et al., 1995). The combined electrodes were included for iontophoretic drug applications; however, no drugs were applied during the experiments reported here. The electrodes were assembled in two different configurations within a stainless steel guide: A circular configuration included two regular and two combined electrodes, with interelectrode distances of 300–600 µm and a linear configuration of four regular electrodes distanced 350 1/2 32 µm from each other. Using a microdrive system, designed and built by Y. de Ribaupierre (The Institute of Physiology, University of Lausanne, Lausanne, Switzerland), the microelectrode array was brought close to the exposed dura, and each of the four electrodes was introduced independently into the somatosensory cortex at a site chosen according to stereotaxic coordinates. The electrodes were introduced perpendicular to the surface of the cortex and to a depth that corresponded to layer IV. The location of each penetration was determined in relation to the bregma by direct measurements with an ocular-micrometer and by mapping on an enlarged photograph of the superficial blood vessels of the particular brain being studied. For each penetration, local field potentials (LFPs) and up to 16 single- and/or multi-units were recorded simultaneously from four electrodes during spontaneous activity and during response to mechanical stimulation of vibrissae or head surfaces. Each electrode channel was amplified (310,000–40,000) and filtered (.5–10 kHz) and then fed into a template-matching spike sorter (MSD-2, AlphaOmega Engineering, POB 2091, Nazareth, Israel). The amplified signals were also fed into a loudspeaker through VIBRISSAL AND BARREL ARRANGEMENT IN RODENTS an offset circuit that removed background noise. To determine the principal vibrissae associated with the recording sites of the different electrodes, different vibrissae and the skin of the head were stimulated with a thin hand-held probe while the population responses were estimated by listening to the loudspeaker. When a responding vibrissa was ascertained, it was inserted into a small teflon tubing attached via a probe to a linear-displacement motor (Schneider, 1988) that was controlled by the experimental computer. Vibratory, square-wave stimuli (80–320 µm p-p, 1–20 Hz; see Fig. 6a) were applied to the selected vibrissa(e). Receptive field boundaries were determined according to the peri-stimulus-time-histograms (PSTHs) of the neuronal responses. Upon completion of the extracellular recordings and receptive field determinations, the sites of recording were marked by passing anodal DC currents through the electrodes, which resulted in tissue coagulation and were used for the reconstruction of the map of penetrations in a given experiment. Preparation of mystacial pad samples After excision of whiskerpad, the vibrissae and intervibrissal hairs (fur) were cut close to the skin. Excised whiskerpads were placed into a simple flattening device that consisted of a polished stainless steel base with two fixed screws, a piece of sponge, and a cover that fit over the base. The whiskerpad (skin-side down) was placed on the polished surface of the base, covered with the sponge, and pressed with the cover using two nuts. The filled flattening device was placed in Carnoy’s fluid overnight, then transferred sequentially through ethyl alcohol solutions (70%, 90%, 95%, and 100%; 1 hour in each), taken out of the flattening device, and placed overnight in xylene for clearing. By the next day, the whiskerpads had become transparent, except for the vibrissal sinus regions, and were photographed. The tissue preparations were then placed in paraffin at 56°C. After 2 hours, the paraffin-soaked tissue was again placed into the flattening device, which was also in the chamber maintained at 56°C, flattened, and immediately dipped into ice-cold water. The flattened, paraffin-soaked tissue was attached to a wooden platform, and sections (40 or 100 µm thick) were prepared using a microtome. These sections were stained with cresyl violet, washed, stained supplementary with thiazine red, and mounted. The thicker sections (100 µm) were used to determine the intrinsic musculature. Processing of cerebral cortex preparations Excised brain hemispheres were cut sagittally into two symmetrical pieces. The cerebral cortex was carefully separated from the rest of the brain and placed into a flattening device similar to that used to flatten whiskerpads. The outer surface of the cortex was placed on the polished surface of the base, the subcortical white matter was covered with a sponge, and the sample was gently pressed, as described above for the mystacial pads. Flattened cortical tissues were embedded in paraffin and sectioned in a fashion similar to that used for the whiskerpads. Flattened cortices were cut parallel to the pial surface overlying the PMBSF. Some of the guinea pigs brains were sectioned also in coronal (see Fig. 4c) and oblique planes oriented posteromedial to anterolateral, as recommended by Chmielowska et al. (1989) for the rat cortex, in order to produce sections ‘‘along’’ the barrel rows. 517 Serial histological sections were examined for PMBSF, electrode tracks, and/or sites of coagulation. In a group of six randomly selected guinea pigs, a morphometric analysis was performed to compare two populations of cortical barrels which represent mystacial and nasal vibrissae. From studying the shape of the barrels in tangential, coronal, parasagittal, and oblique Nissl-stained sections, we concluded that the configuration of the barrels in the PMBSF approximately resembles an ellipsoid, of which vertical axes that correspond to the thickness of layer IV are approximately the same for all the barrels. So, the differences calculated for the mean area of a barrel in different groups of barrels measured in tangential sections would reflect the differences in barrel volumes. Barrel area was calculated according to the formula of an ellipse: S 5 pab, where a is the large, and b is the small radius of a barrel. Barrel radii were measured from the calibrated camera lucida drawings of the PMBSF. Mean areas were calculated for the four posterior barrels representing the four largest nasal vibrissae and for the barrels corresponding to each of the 24 usually present mystacial vibrissae (four straddlers, two vibrissae in row A, three in row B, and five in each of the rows C, D, and E). Statistical significance was estimated using a two-sided Student t-test. In two guinea pigs and two golden hamsters, brain tissues were also processed for CO histochemistry. The animals were perfused transcardially with 2.5% glutaraldehyde, 0.5% paraformaldehyde, and 5% sucrose in 0.1 M sodium phosphate (PO4) at pH 7.4. Freezing microtome sections (60 µm) were prepared and left overnight in perfusion solution at 4°C. The next day, sections were incubated in oxygenated solution of 4% sucrose, 0.02% cytochrome C (Type VI, Sigma), 0.05% diaminobenzidine in PO4 for 2–3 hours at 37°C under constant agitation (Land and Simons, 1985). The reaction was terminated by PO4 change. CO-stained sections were mounted on gelatincoated slides, air-dried, and coverslipped using Entellan. Production of photomicrographs Photomicrographs were taken by using Wild Makroskop M 420 with reversal Kodak Ektachrome 160T or negative black-and-white T-MAX 100 35-mm professional film. Figures 3 and 8 were printed by using standard darkroom methods. Figures 1, 2, 4, 5, 6a and c, 7, and 8a and c were processed by a computer. Original photographic color slides (for Figs. 2, 4, and 5) were scanned on a Kodak Professional RFS 2035 Plus Film Scanner, and black-andwhite prints were scanned on a Nicon AX-1200 scanner. Image editing was performed on a Power Macintosh 9500/132 with Adobe Photoshop 3.0 using standard tools. Only the sharpness, contrast, and/or brightness were adjusted. Final prints were prepared on a Kodak XLS 8600 PS printer. RESULTS Vibrissal arrangement and nomenclature In cleared mystacial preparations, all large and small sinus hairs on the face of the guinea pig and golden hamster were easily observed (Fig. 1). Almost all the mystacial vibrissae of these rodents were arranged in essentially straight rows. The sizes of the vibrissae diminished from the caudal to rostral direction. The most caudal arc was composed of four large mystacial vibrissae that Fig. 1. Vibrissal arrangement in the mystacial pad of the guinea pig (A) and golden hamster (B). Darkfield photographs of entire whiskerpads after clearing in xylene; all sinus hairs are visualized. A clear arrangement of mystacial vibrissae in five rows (A–E) that are oriented from caudal to rostral along the snout is observed. The four most caudally placed large vibrissae (a, b, g, and d) are positioned in the spaces that would be between the rows if the rows were continued caudally. These four vibrissae straddle vibrissal rows from A to E. C, caudal; FBP, furry buccal pad; N, nasal vibrissae; NS, nostril; R, rostral, V, ventral. Scale bars 5 1 mm. VIBRISSAL AND BARREL ARRANGEMENT IN RODENTS were not situated in the essentially straight rows. These vibrissae were in the middle of the spaces formed by an imaginary continuation of the vibrissal rows in the caudal direction and thus could be considered as straddlers. To verify that these vibrissae are straddlers, we studied the intrinsic musculature of the mystacial pad in thick (100-µm) sections of the mystacial pad (Fig. 2). In vibrissal rows of both guinea pigs and golden hamsters, the intrinsic muscles were only connecting the neighboring vibrissae of the same row. Each of the most caudal four vibrissae was connected via follicular muscles with two rows of mystacial vibrissae. If we apply the nomenclature proposed by Van der Loos and Woolsey (1973) for vibrissae classification in mice and rats, the intrinsic muscles would connect straddler a with rows A and B, straddler b with rows B and C, straddler g with rows C and D, and straddler d with rows D and E. Intrinsic muscles that would connect the straddlers with the most dorsal row of sinus hairs outside the mystacial pad (nasal vibrissae) were not observed. This pattern of muscle distribution suggests that guinea pigs and golden hamsters possess four straddlers and five rows of mystacial vibrissae, which are typical for mice and rats, and that the most dorsal row of facial vibrissae of the guinea pig and golden hamster does not share the same structural and functional relationships that exist between the other five rows of facial vibrissae and the straddlers, and thus should not be considered as a part of the mystacial vibrissae. In the guinea pig, the anterior part of this nonmystacial vibrissal row, which is located dorsally to the horizontal line crossing the nostrils, was previously called nasal vibrissae (Cooper and Schiller, 1975). We believe that these nasal vibrissae have a caudal continuation that was previously considered as row A of mystacial vibrissae (Sikich et al., 1986). In the golden hamster, the most dorsal row of facial vibrissae appears similar to that of the row of nasal vibrissae in the guinea pig (Fig. 1b). To further compare the features of the tissue containing facial vibrissae, we studied the behavior of the mystacial pad during tissue processing. The clearing procedure provided additional evidence that the sinus hairs of the most dorsal row are not part of the mystacial pad of the guinea pig and golden hamster. This dorsal row, which includes four to eight large sinus hairs, became clearly visible at the beginning of clearing procedure, whereas the mystacial pad, which is relatively rich in muscles and connective tissues, only became transparent later (Fig. 3). Whether the peculiar behavior during tissue processing of the whisker follicles in this dorsal row might simply be related to the thickness of the tissue was checked by flattening the snout specimens during fixation and dehydration without using a sponge. The specimens thus flattened were of even thickness. They were taken out of the flattening device and dipped into xylene. During the clearing procedure, the mystacial pad still remained opaque for at least 1 or 2 hours after the tissue around the nasal vibrissae had become transparent. Thus, the differential clearing characteristics were a function of the type of tissue, not of possible variations in the thickness of the tissue. Although the fundamentals, with regard to straight rows and straddlers, of the spatial organization of vibrissae for guinea pig were similar to those in mice and rats, the precise arrangement of vibrissae, especially straddlers, differed. In guinea pigs, the vertical distances between the vibrissal rows were less than the diameters of 519 the straddlers bulbs. As a result, the straddlers were arranged in a semicircular manner, with the d-straddler located more rostral and appearing like a continuation of the arc formed by vibrissae A1, B1, and C1 (see Figs. 1a, 2a). Vibrissae D1 and E1, which were straddled by this d-straddler, appeared like a continuation of the arc formed by vibrissae A2, B2, and C2. In the golden hamster, the straddler arrangement appeared the same as in mice and rats, but the row of nasal vibrissae and row A of mystacial vibrissae were overlapping: One or two of the most caudal nasal vibrissae were located dorsal to row A, whereas the rest of the nasal vibrissae were shifted ventrally, forming an apparent rostral continuation of row A (see Figs. 1b, 2b). Thus, based on these three observations [(i) a pattern of vibrissa arrangement that is consistent with four straddlers and five rows; (ii) specific distribution of intrinsic musculature in the mystacial pad; and (iii) peculiar behavior of the mystacial pad during tissue processing], we conclude that the mystacial pads of the guinea pig and golden hamster indeed contain four straddlers that straddle five rows of mystacial vibrissae, and that the most dorsal row of facial vibrissae is located outside the mystacial pad and should be considered as nasal vibrissae. We propose that the short second row of facial vibrissae be designated as row A of mystacial vibrissae. Ventral to this row A is a row of mystacial vibrissae we designate as row B. In the guinea pig, this row B usually contained three, and in the golden hamster, four vibrissae, just like row B in rats which also contains four. In the guinea pig and golden hamster, as in mice and rats, the next three rows of mystacial vibrissae (C, D, and E) were the longest. These rows usually contained four to six large vibrissae and had a rostral continuation that consisted of smaller sinus hairs. Ventral to rows C–E, the upper lips of the guinea pig and golden hamster were also covered with small sinus hairs, which were arranged in five rows. Similar hairs were designated as labial sinus hairs in mice (Dörfl, 1982) and as rows F, G, H, I, and J in rats (Brecht et al., 1997). From the lateral part of the upper lip, the small sinus hairs appeared to pass onto the inner surface of the upper lip (see Fig. 1), thus forming a furry buccal pad (FBP) similar to the one described in the rat (Welker, 1976). In both guinea pigs and golden hamsters, the FBP was represented in the somatosensory cortex anterior to the PMBSF. When comparing the described patterns of vibrissa arrangement in the guinea pig and golden hamster with those of mice and rats, a striking similarity emerges. This similarity further supports our proposal and provides support for the same principles of classification and nomenclature to be used in these four species of rodents. Structural representation of the vibrissae in the cortex The patterns of barrel arrangement, including the PMBSF, in sections of cerebral cortices of the guinea pig and golden hamster were examined. In Nissl- and COstained sections of the cortex cut parallel to the surface of the flattened cortex, the PMBSF was composed of visible spheroidal barrels arranged in five rows that were directed postero-medial to rostro-lateral (Figs. 4a,b, 5). In the guinea pig, usually, two large barrels were present in row A. Row B, which was medio-rostral to row A, usually consisted of three or four barrels in an almost straight line. Parallel to row B, in the medio-anterior direction, were Fig. 2. Cresyl violet supplemented with thiazine red staining of thick (100-µm) sections of flattened mystacial pads of the guinea pig (a) and golden hamster (b). The rostral segment of the follicles of each large mystacial vibrissa is surrounded, in a sling-like fashion, by intrinsic muscles (arrows). The ends of the slings are oriented caudally. a, b, g, and d are straddlers; A–E, rows of vibrissae; N, nasal vibrissae; R, rostral; V, ventral. Scale bars 5 1 mm. VIBRISSAL AND BARREL ARRANGEMENT IN RODENTS 521 Fig. 3. Mystacial pads of the guinea pig (a) and golden hamster (b) during incubation in xylene. Centrally located opaque discs correspond to the whiskerpads, while the sinus hairs of the upper lip (UL) and nasal vibrissae (N1–N6) are cleared. a, b, g, and d indicate the locations of the four straddlers, which are not yet cleared. D, dorsal; NS, nostril; R, rostral. Scale bars 5 1 mm. rows C, D, and E, which each contained four to five barrels. These five rows of barrels were at an angle of about 40° to the longitudinal axis of the brain. The row of barrels representing the nasal vibrissae was located laterally to row A and oriented at a bigger angle to the brain axis. Barrels representing the nasal vibrissae were significantly smaller than barrels representing mystacial vibrissae. A morphometric analysis has shown that the mean area of a barrel corresponding to mystacial vibrissae was 64 · 103 6 7 · 103 µm2, whereas that of a barrel corresponding to nasal vibrissae was 28 · 103 6 6 · 103 µm2 (P , .001). In sections of the guinea pig cortex cut perpendicularly to the surface of the cortex, and especially in coronal sections (Fig. 4c,d), barrels were also easily visualized, and possessed a spheroidal, rather than the dubbed shape previously reported for rodents and some lagomorphs (Woolsey and Van der Loos, 1970; Weller, 1972; Woolsey et al., 1975). In Nissl-stained sections of the flattened cortex of the golden hamster cut parallel to pial surface, the PMBSF was composed of five rows of hollow barrels, arranged in a fashion demonstrated previously by Woolsey et al. (1975) with thionin-stained sections. Because the hollow barrels of the golden hamster were less prominent than those of the guinea pig and occupied only the deeper part of layer IV in the barrel field, the entire PMBSF could not be clearly seen in a single Nissl-stained tangential section; thus several cortical sections were used to prepare camera lucida drawings (see Fig. 8d below). These peculiarities of the golden hamster barrels were previously mentioned by Rice et al. (1985) and Rice (1995). In CO-stained tangential sections, we could obtain all the barrels of the PMBSF in a single section (Fig. 5). Previous reports seem to demonstrate an incomplete picture in 2-DG- and CO-labeled tangential sections through layer IV of the hamster barrel cortex (Jacquin et al., 1993). Electrophysiological verification of the cortical representation of the mystacial vibrissae Cortical barrels were related to specific vibrissae by mapping the receptive fields of single neurons and of groups of neurons. Multiple penetrations at intervals of about 300 µm, starting from the site where the response to straddler stimulation was observed, were performed. Stimulation of a single vibrissa usually evoked response in only one, and in some cases in two, of the electrodes introduced into the PMBSF. This enhanced neuronal activity was evident in the unfiltered electrode signal feeding the loudspeaker, in the LFP and multi-unit responses, and was usually evident in the response of at least one of the single units recorded. Responses of multi-units, simultaneously recorded from four electrodes that were arranged in a linear array, are demonstrated in Figure 6. The units were recorded from layer IV, and the recording sites were marked by electrolytic lesions (Fig. 6b). The PSTHs (Fig. 6a) show a clear functional segregation between the cortical barrels. The neurons recorded from the g-barrel (el. 1) responded to stimulations of vibrissae g and C1 and not to stimulations of vibrissae B1 or A2. C1-barrel neurons (el. 2) responded only to vibrissa C1, neurons recorded from 522 Fig. 4. Nissl (a,c) and cytochrome oxidase (b,d) staining of the sections (60 µm) of cerebral cortices of guinea pigs. Tangential sections through the posteromedial barrel subfield (PMBSF) of flattened cortices of right hemispheres (a,b), and coronal sections through the PMBSF of left hemispheres (c,d). Roman numerals demarcate cortical S. HAIDARLIU AND E. AHISSAR laminae. Labels in a and b are centered inside the barrels. a, b, g, and d represent straddlers; A–E, rows of mystacial vibrissae; N, nasal vibrissae; SO, supraorbital vibrissa. White arrowheads point to barrels; black arrowheads indicate the loci of lesions. M, medial; R, rostral; W, white matter. Scale bars 5 1 mm. VIBRISSAL AND BARREL ARRANGEMENT IN RODENTS 523 Fig. 5. Cytochrome oxidase staining of a tangential section (60 µm) through layer IV of the hamster left barrel cortex. Conventions and abbreviations as in Figure 4. the border between barrels B1 and B2 (el. 3) responded to both vibrissae B1 and B2 (the latter is not shown), and A2-barrel neurons (el. 4) responded to both vibrissae A2 and B2 (the latter is not shown). Note that most of the responses depended upon the direction of vibrissa movement (retraction vs. protraction, lower trace). Both spatial segregation and directional dependencies were typical for all PMBSF recordings. Another example of the observed spatial segregation of cortical responses is depicted in Figure 7. In this figure, the average firing rates of two single-units, recorded from layer IV during the penetrations marked in Figure 4b, are plotted as a function of time. During the simultaneous stimulation of straddler b and vibrissa B1, the firing rates of both neurons were significantly increased. However, when vibrissa B1 was stimulated alone, only the neuron recorded near barrel B1 (el. 4) was activated. Figure 8 summarizes the spatial arrangements of the sinus hairs on the snouts of the guinea pig (Fig. 8a) and golden hamster (Fig. 8c) and the spatial arrangements of the barrels in the PMBSF of these two species (Fig. 8b and d, respectively). This figure demonstrates the proposed adaptation, for these two species, of the nomenclature used for the vibrissa arrangement of the mouse and rat (Van der Loos and Woolsey, 1973). In the rostral part of the barrel field (data not shown), both the vibrissal follicles and the hairs that surround them were represented, which is similar to the situation in mice (Nussbaumer and Van der Loos, 1985). DISCUSSION In order to understand the general principles of how structures in the periphery generate topographic maps at higher levels of the nervous system, and how these representations interconnect to form processing networks, a detailed description of such systems in a variety of mammals is essential. Such descriptions will elucidate similarities in peripheral and cortical organization, as well as differences that exist as a result of different location of specialized receptor structures on the sensory surface, and the function or use of such structures. This study was initiated to (i) delineate the general patterns of vibrissa and barrel arrangement in guinea pigs and golden hamsters, (ii) determine the cortical representation of each whisker of the whiskerpad of these two species, and (iii) compare the general organizational principles of the whiskerpad-PMBSF system of the guinea pig and golden hamster with those of the mouse and rat. A highly reproducible map of both the vibrissal distribution in the mystacial pad and of the barrel arrangement in the PMBSF of the guinea pig and golden hamster was obtained, and their functional correlation was determined. These findings support application to the guinea pig and golden hamster 524 S. HAIDARLIU AND E. AHISSAR Fig. 7. Effect of vibrissa stimulation on the firing rates of two individual neurons in the PMBSF of the guinea pig. Neuronal activities were recorded simultaneously by electrode 3 located in barrel b and electrode 4 which was touching barrel B1 (see Fig. 4b). Thick traces indicate the average firing rates of the single-units, recorded from layer IV, as a function of time (bin 5 10 seconds). Thin traces indicate a moving estimation of the mean 1/2 2*std, computed for every data point from its consequent 30 bins, not including the stimulation periods. Last 30 bins of the records were duplicated for this computation. Thick horizontal bars indicate the time of stimulation of the vibrissae. Stimuli were continuous square-wave vibratory movements at 5 Hz and 160 µm p-p (see Fig. 6a, bottom trace). Fig. 6. Effect of rhythmic vibratory stimulation (5 Hz) of individual whiskers from the left side of the face on firing probabilities of multi-units recorded simultaneously from different barrels of the right PMBSF of a guinea pig. a: peri-stimulus-time-histograms (PSTHs; 1/2 100 ms, bin 1 ms) were computed from continuous stimulus cycles for the multi-units recorded from each of the electrodes (el. 1–4) and for each stimulated vibrissa. A single stimulus cycle is depicted at the bottom trace. Number of stimulus cycles: g, 288; C1, 289; B1, 272; A2, 289. All PSTHs sharing the same rows have the same scale. b: A camera lucida drawing of the PMBSF of the same animal is shown. Electrolytic lesions were produced by delivering a DC current between the tips of the recording electrodes and the ground electrode. After subsequent staining for cytochrome oxidase, the PMBSF was reconstructed from 5 consecutive 60-µm-thick sections. Arrowheads indicate the loci of lesions. Labels are located inside the barrels. M, medial; R, rostral. Abbreviations and conventions as in Figure 4. Scale bar 5 1 mm. of the nomenclature for vibrissal and barrel arrangement previously elaborated in mice and rats (Van der Loos and Woolsey, 1973). Relation to previous findings Histochemical delineation of the forth layer in the somatosensory cortex of the guinea pig was first reported by Friede (1960). The general organization of the somatic afferent areas, including the representation of the mystacial pads, in the cortex of the guinea pig was illustrated by Zeigler (1964), and Zeigler’s data were confirmed by Campos and Welker (1976) and Rapisarda et al. (1990). Unlike the vigorous whisking of rats and mice, guinea pigs move their vibrissae in brief irregular bursts (Rice, 1995). This might be the reason why only few studies examined the guinea pig’s vibrissa/somatosensory system. Upon discovery of barrels in the somatosensory cortex of the mouse (Woolsey and Van der Loos, 1970) and their precise description in the rat (Welker, 1971; Welker and Woolsey, 1974), the presence of such barrels in other experimental species of rodents was examined. Hollow barrels were observed in the guinea pig (Woolsey et al., 1975; Rice et al., 1985), but the exact map and the nomenclature of the PMBSF was not obtained. Sikich et al. (1986) stated that the appropriate electrophysiological and lesion studies, which were needed to definitively identify individual architectonic units as the representations of specific vibrissae, had not been performed in guinea pigs. These authors concluded, however, that the arrangement of vibrissae on the face and the patterns and locations of the barrels in the central nervous system of the guinea pig differed from those of mice and rats in that (i) there were six rather than five rows of mystacial vibrissae and of vibrissal representations and (ii) the rows were more irregularly aligned. In the present study, we observed that the guinea pig has four straddlers and that the most dorsal row of the previously suggested six rows of mystacial vibrissae differs from the other five rows by (i) not being straddled (Figs. 1a, 8a), (ii) not being connected via follicular muscles to a straddler or to other vibrissa in the mystacial pad (Fig. 2a), (iii) responding differently to the clearing procedure (Fig. 3a), which indicates a different histological structure, (iv) being located dorsal to the horizontal line crossing the nostril, and (v) being represented by cortical barrels that are significantly smaller than those representing the other five rows, and that are often oriented in a different direction (Fig. 4a,b). Furthermore, the apparent irregularity in the orientation of both the vibrissae and barrels VIBRISSAL AND BARREL ARRANGEMENT IN RODENTS 525 Fig. 8. Mapping of the sinus hairs onto the posteromedial barrel subfield. Photomicrographs of unstained cleared mystacial pads of the guinea pig (a) and golden hamster (c). The rows of vibrissae are marked A–E in the dorsal to ventral direction and numbered caudal to rostral. The straddlers, which are designated a, b, g, and d, straddle rows A–B, B–C, C–D, and D–E, respectively, of vibrissae. In the camera lucida drawings of the corresponding barrels in the PMBSF of the guinea pig (b) and golden hamster (d), barrels are represented as irregular elliptical figures of appropriate shapes and dimensions. The barrels that correspond to the straddlers form one arc, which starts at the caudo-lateral edge and is directed medio-rostrally along the posterior border of the PMBSF. M, medial; N, nasal vibrissae; NS, nostril; P, posterior; R, rostral; V, ventral. Scale bars 5 1 mm. disappears if the presence of four straddlers is considered. With this consideration, four straddlers and five almost straight rows of vibrissae are clearly seen in the whiskerpad of the guinea pig. In light of the presence of these four straddlers, the number of vibrissae cited by Sikich et al. (1986) for each row should be revised. Unlike the guinea pig, the golden hamster is a typical whisking rodent. Nevertheless, its vibrissae/somatosen- sory system was not studied in detail. By measuring the distances between adjacent vibrissae of the golden hamster under a dissecting microscope and plotting a grid map for them on a diagram of the head (Wineski, 1983), Wineski (1985) reported that in the golden hamster, vibrissae are organized in a gridlike pattern of seven rows and seven columns along the anteroposterior and dorsoventral planes of the snout. This appeared to contradict a previous 526 indication that the hamster’s vibrissae are represented in the somatosensory cortex by only five rows of barrels (Woolsey et al., 1975). Nevertheless, Wineski (1983, 1985) developed new classification principles for vibrissae mapping, in which all the hamster vibrissae are arranged in seven rows (from A to G) in anteroposterior direction of the mystacial pad and seven columns (1 to 7) from the dorsal to ventral direction, with no allowances made for straddlers. In the golden hamster, as in the guinea pig, we also clearly demonstrated four straddlers and five rows of mystacial vibrissae. The most dorsal row of facial vibrissae (sometimes considered as two rows, e.g., Wineski, 1985) has a rostral continuation and is a row of nasal vibrissae. We demonstrate the same distribution of barrels in the PMBSF. These results are in agreement with Woolsey et al., (1975) and with previous CO histochemistry in the hamster which also demonstrated only five rows of barrels in the PMBSF (Jacquin et al., 1993). Why were nasal vibrissae confused with mystacial ones in previous studies? We believe that the main reason involves the inconsistent pattern of distribution of nasal vibrissae among different rodents. There seem to be at least two different patterns: an elliptic arrangement of four or more vibrissae dorsal to the nostril that is characteristic for mice and rats, and an almost straight horizontal row of four to eight vibrissae that is characteristic for guinea pigs and golden hamsters. In these latter rodents, the nasal vibrissae row starts dorsal to vibrissa A1 (in the guinea pig) or close to vibrissa A2 (in the golden hamster), and finishes dorsal to the nostril in both species. Why were straddlers overlooked previously in guinea pigs and hamsters? The reasons are probably different for the two species. In the guinea pig, the straddlers are not arranged in a straight line, because of their large dimensions. As a result, their straddling position is not evident from the position on the skin but rather from the arrangement of the intrinsic musculature of the mystacial pad (see Fig. 2). In the hamster, the spaces between the vibrissal rows are so small that straddlers can be easily brought in line with the corresponding rows of vibrissae. The barrels corresponding to straddlers are very large and barely delineated, especially in Nissl-stained sections, and can be easily overlooked. Comparison of the vibrissae/somatosensory systems of the guinea pig, golden hamster, mouse, and rat The presence of straddlers and the arrangement of vibrissae in five almost straight rows in the mystacial pads of guinea pigs, hamsters, mice, and rats support a similar vibrissal distribution. Mapping of the receptive fields of the vibrissae-associated barrels in these species also corroborates this similarity. Furthermore, three vibrissal rows (C, D, and E) in the guinea pig (Fig. 1a), golden hamster (Fig. 1b), and rat (Welker, 1971) have a direct rostral continuation that is composed of small nonvibrissal sinus hairs. The distribution of intrinsic muscles in the mystacial pad of guinea pigs, golden hamsters, and mice is also similar. In guinea pigs, as in mice (Van der Loos and Dörfl, 1978), an extra vibrissa can occasionally be found in the mystacial pad. In only two of the 41 guinea pigs studied was an additional vibrissa observed. This small extra vibrissa, which was rostro-ventrally to row A and on the apparent border of the mystacial pad (Fig. 1a, not labeled), was S. HAIDARLIU AND E. AHISSAR represented in the PMBSF by an additional barrel in row A. In the golden hamster, a third vibrissa in row A was seen in over half the preparations. The vibrissal barrel field of the mouse can be divided into compartments (Nussbaumer and Van der Loos, 1985): the PMBSF, which receives only vibrissal input, and an anterior part, which is populated with hollow barrels, and receives inputs from vibrissal follicles, intervibrissal hairs, and skin. In the present study, similar compartments were observed in the guinea pig and golden hamster. Are those structural similarities accompanied by functional similarities? At least for the rat and the guinea pig, it seems to be the case. In both species, many neurons in the somatosensory cortex exhibit spontaneous oscillations around 10 Hz, which can be entrained by periodic vibrissal stimulations between, usually, 3 and 12 Hz (Ahissar et al., 1996). The functional details characterizing both the spontaneous and the entrained neuronal behaviors appeared to be similar in rats and guinea pigs. These similarities suggest that the difference in whisking behavior between these two species is probably mainly due to motor, rather than sensory, differences. Furthermore, these structural and functional similarities should constrain models assigning a critical role for development in the formation of cortical barrels or other sensory modular organizations. Difficulties encountered during determination of the arrangement of vibrissae and cortical barrels During determination of the vibrissal arrangement in the whiskerpad of rodents, difficulties arise if the positions of the vibrissae change during processing due to stretching, excessive flattening etc. We found that proper flattening and paraffin-embedding of the mystacial pad preserved the actual positions of the vibrissae for visualization. During transformation of the convex surface of the mystacial pad into a planar one by our new technique for flattening and embedding, the artifacts that appeared were minimal. The dimensions of the sinus hairs and the direction of the vibrissal axes must be taken into account when determining the section thickness and cutting plan. The clarity of vibrissal representation in the PMBSF is limited by vertical displacements of some barrels (see the arrowheads in Fig. 4c,d), in addition to the usual limitations of histological procedures. In tangential sections, these vertically displaced barrels were barely seen, because their hollows did not lie in the plane of the single section. The differential appearance of the Nissl-stained barrels in row E affected the imaging of barrels. In some cases, the hollows of the barrels E1 and E2 were not evident, whereas the hollows of E3 and E4 were always clearly evident. However, staining for CO is providing a better imaging of the PMBSF including all the barrels of row E. The variability in the distribution of the small barrels that represent the nonmystacial row of sinus hairs located dorsally to the whiskerpad is high, with frequent extra barrels and variations in the location of the area occupied by their representation. CONCLUSIONS The organizational principles of, and the correspondences between, the vibrissae in the mystacial pad and the barrels in the PMBSF of the guinea pig and golden VIBRISSAL AND BARREL ARRANGEMENT IN RODENTS hamster are similar to those of the mouse and rat. Two templates of vibrissa arrangement for the nasal vibrissae, one characteristic for the guinea pig and golden hamster and one characteristic for mice and rats, may account for seemingly contradictory classifications previously proposed. The differences in the neuronal mechanisms that are associated with the different motor patterns of vibrissal touch possessed by guinea pigs compared to hamsters, mice, and rats are not reflected in the anatomical structures, proposing that the differences in whisking patterns originate from functional and not structural differences. ACKNOWLEDGMENTS We thank Drs. M. Segal, R. Malach, and B. Schick for helpful comments on this manuscript. We are also grateful to M. Harel for her skilled CO histochemistry, and to K.O. Johnson for kindly providing the vibrotactile stimulator. This work was supported by the Minna-James Heinemann Foundation, Germany; the Minerva Foundation, Munich, Germany; and by grant 93-00198 from The US-Israel Binational Science Foundation, Israel. S.H. was supported by the Gileadi program, Ministry of Absorption, Israel, and E.A. was supported by an Alon Fellowship, Israel. LITERATURE CITED Ahissar, E., G. Alkon, M. Zacksenhouse, and S. Haidarliu (1996) Cortical somatosensory oscillators and the decoding of vibrissal touch. Soc. Neurosci. Abstr. 22:18. Brecht, M., B. Preilowski, and M.M. Merzenich (1997) Functional architecture of the mystacial vibrissae. Behav. Brain Res. 84:81–97. Campos, G.B., and W.I. Welker (1976) Comparisons between brains of a large and a small hystricomorph rodent: Capibara, Hydrochoerus and guinea pig, Cavia; neocortical projection regions and measurements of brain subdivisions. Brain Behav. Evol. 13:243–266. Chmielowska, J., G.E. Carvell, and D.J. Simons (1989) Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J. Comp. Neurol. 285:325–338. Cooper, G., and A.L. Schiller (1975) Anatomy of the Guinea Pig. Cambridge, MA: Harvard University Press. Dörfl, J. (1982) The musculature of the mystacial vibrissae of the white mouse. J. Anat. 135:147–154. Friede, R.L. (1960) A comparative study of cytoarchitectonics and chemoarchitectonics of the cerebral cortex of the guinea pig. Z. Zellforsch. 52:482–593. Haidarliu, S. (1996) An anatomically adapted, injury-free headholder for guinea pigs. Physiol. Behav. 60:111–114. Haidarliu, S., D. Shulz, and E. Ahissar (1995) A multielectrode array for 527 combined microiontophoresis and multiple single-unit recordings. J. Neurosci. Methods 56:125–131. Jacquin, M.F., J.S. McCosland, T.A. Henderson, R.W. Rhoades, and T.A. Woolsey (1993) 2-DG uptake patterns related to single vibrissae during exploratory behaviors in the hamster trigeminal system. J. Comp. Neurol. 332:38–58. Land, P.W., and D.J. Simons (1985) Cytochrome oxidase staining in the rat SmI barrel cortex. J. Comp. Neurol. 238:225–235. Nussbaumer, J.-C., and H. Van der Loos (1985) An electrophysiological and anatomical study of projections to the mouse cortical barrelfield and its surroundings. J. Neurophysiol. 53:686–698. Rapisarda, C., A. Palmeri, G. Aicardi, and S. Sapienza (1990) Multiple representations of the body and input-output relationships in the agranular and granular cortex of the chronic awake guinea pig. Somatosens. Res. 7:289–314. Rice, F.L. (1995) Comparative aspects of barrel structure and development. In E.G. Jones and I.T. Diamond (eds): Cerebral Cortex, Vol. 11. The Barrel Cortex of Rodents. New York: Plenum Press, pp. 1–75. Rice, F.L., C. Gomez, C. Barstow, A. Barnet, and P. Sands (1985) A comparative analysis of the development of the primary somatosensory cortex: interspecies similarities during barrel and laminar development. J. Comp. Neurol. 236:477–495. Schneider, W. (1988) The tactile array stimulator. Johns Hopkins APL Technical Digest 9:39–43. Sikich, L., T.A. Woolsey, and E.M. Johnson (1986) Effect of a uniform partial denervation of the periphery on the peripheral and central vibrissal system in guinea pig. J. Neurosci. 6:1227–1240. Van der Loos, H., and J. Dörfl (1978) Does the skin tell the somatosensory cortex how to construct a map of the periphery? Neurosci. Lett. 7:23–30. Van der Loos, H., and T.A. Woolsey (1973) Somatosensory cortex: structural alterations following early injury to sense organs. Science 179:395–398. Welker, C. (1971) Microelectrode delineation of fine grain somatotopic organization of SmI cerebral neocortex in albino rat. Brain Res. 26:259–275. Welker, C. (1976) Receptive fields of barrels in the somatosensory neocortex of the rat. J. Comp. Neurol. 166:173–190. Welker, C., and T.A. Woolsey (1974) Structure of layer IV in the somatosensory neocortex of the rat: description and comparison with the mouse. J. Comp. Neurol. 158:437–454. Weller, W.L. (1972) Barrels in somatic sensory neocortex of the marsupial Trichosurus vulpecula, brush-tailed opossum. Brain Res. 43:11–24. Wineski, L.E. (1983) Movements of the cranial vibrissae in the Golden hamster (Mesocricetus auratus). J. Zool., Lond. 200:261–280. Wineski, L.E. (1985) Facial morphology and vibrissal movement in the golden hamster. J. Morphol. 183:199–217. Woolsey, T.A., and H. Van der Loos (1970) The structural organization of layer IV in the somatosensory region (S1) of the cerebral cortex: the description of a cortical field composed of discrete cytoarchitectonic units. Brain. Res. 17: 205–242. Woolsey, T.A., C. Welker, and R.H. Schwartz (1975) Comparative anatomical studies of the Sm1 face cortex with special reference to the occurrence of ‘‘barrels’’ in layer IV. J. Comp. Neurol. 164:79–94. Zeigler, H.P. (1964) Cortical sensory and motor areas of the guinea pig (Cavia Porcellus). Arch. Ital. Biol. 102:587–598.