* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ch.05The Structure and Function of Large Biological Molecules
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Gene expression wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Epitranscriptome wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Blood sugar level wikipedia , lookup
Peptide synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Point mutation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
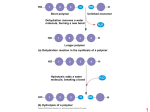
HO 1 2 3 H Short polymer HO Unlinked monomer Dehydration removes a water molecule, forming a new bond HO 1 H 2 3 H 2O 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3 4 Hydrolysis adds a water molecule, breaking a bond HO 1 2 3 H H H 2O HO H (b) Hydrolysis of a polymer 1 Aldoses Trioses (C3H6O3) Pentoses (C5H10O5) Hexoses (C6H12O6) Glyceraldehyde Ribose Ketoses Glucose Galactose Dihydroxyacetone Ribulose Fructose 2 (a) Linear and ring forms (b) Abbreviated ring structure 3 1–4 glycosidic linkage Glucose Glucose Maltose (a) Dehydration reaction in the synthesis of maltose 1–2 glycosidic linkage Glucose Fructose Sucrose (b) Dehydration reaction in the synthesis of sucrose 4 Chloroplast Mitochondria Glycogen granules Starch 0.5 µm 1 µm Glycogen Amylose Amylopectin (a) Starch: a plant polysaccharide (b) Glycogen: an animal polysaccharide 5 (a) α and β glucose ring structures α Glucose (b) Starch: 1–4 linkage of α glucose monomers β Glucose (b) Cellulose: 1–4 linkage of β glucose monomers 6 Cell walls Cellulose microfibrils in a plant cell wall Microfibril 10 µm 0.5 µm Cellulose molecules β Glucose monomer 7 8 (a) The structure of the chitin monomer. (b) Chitin forms the exoskeleton of arthropods. (c) Chitin is used to make a strong and flexible surgical thread. 9 Fatty acid (palmitic acid) Glycerol (a) Dehydration reaction in the synthesis of a fat Ester linkage (b) Fat molecule (triacylglycerol) 10 Structural formula of a saturated fat molecule Stearic acid, a saturated fatty acid (a) Saturated fat Structural formula of an unsaturated fat molecule Oleic acid, an unsaturated fatty acid cis double bond causes (b) Unsaturated fat bending 11 Hydrophilic head Hydrophobic tails (a) Structural formula Choline Phosphate Glycerol Fatty acids Hydrophilic head Hydrophobic tails (b) Space-filling model (c) Phospholipid symbol 12 Hydrophilic head Hydrophobic tail WATER WATER 13 14 15 Substrate (sucrose) Glucose OH Fructose Enzyme (sucrase) H2O H O 16 α carbon Amino group Carboxyl group 17 Nonpolar Glycine (Gly or G) Valine (Val or V) Alanine (Ala or A) Methionine (Met or M) Leucine (Leu or L) Trypotphan (Trp or W) Phenylalanine (Phe or F) Isoleucine (Ile or I) Proline (Pro or P) Polar Serine (Ser or S) Threonine (Thr or T) Cysteine (Cys or C) Tyrosine (Tyr or Y) Asparagine Glutamine (Asn or N) (Gln or Q) Electrically charged Acidic Aspartic acid Glutamic acid (Glu or E) (Asp or D) Basic Lysine (Lys or K) Arginine (Arg or R) Histidine (His or H) 18 Peptide bond (a) Side chains Peptide bond Backbone (b) Amino end (N-terminus) Carboxyl end (C-terminus) 19 Groove Groove (a) A ribbon model of lysozyme (b) A space-filling model of lysozyme 20 Antibody protein Protein from flu virus 21 Primary Structure Secondary Structure Tertiary Structure Quaternary Structure β pleated sheet +H 3N Amino end Examples of amino acid subunits α helix 22 Hydrophobic interactions and van der Waals interactions Polypeptide backbone Hydrogen bond Disulfide bridge Ionic bond 23 Polypeptide chain β Chains Iron Heme α Chains Hemoglobin Collagen 24 Normal hemoglobin Primary structure 1 2 3 4 5 6 7 Secondary and tertiary structures β subunit Normal hemoglobin (top view) Val His Leu Thr Pro Val Glu 1 2 3 4 5 6 7 Exposed hydrophobic region β subunit α Quaternary structure β Function Secondary and tertiary structures Sickle-cell hemoglobin β α Quaternary structure Primary structure Val His Leu Thr Pro Glu Glu β Sickle-cell hemoglobin β α Function Molecules do not associate with one another; each carries oxygen. Molecules interact with one another and crystallize into a fiber; capacity to carry oxygen is greatly reduced. 10 µm Red blood cell shape Normal red blood cells are full of individual hemoglobin moledules, each carrying oxygen. α 10 µm Red blood cell shape Fibers of abnormal hemoglobin deform red blood cell into sickle shape. 25 Denaturation Normal protein Renaturation Denatured protein 26 Polypeptide Correctly folded protein Cap Hollow cylinder Chaperonin (fully assembled) Steps of Chaperonin 2 The cap attaches, causing the 3 Action: cylinder to change shape in such a way that it creates a 1 An unfolded polyhydrophilic environment for peptide enters the cylinder from one end. the folding of the polypeptide. The cap comes off, and the properly folded protein is released. 27 DNA 1 Synthesis of mRNA in the nucleus mRNA NUCLEUS CYTOPLASM mRNA 2 Movement of mRNA into cytoplasm via nuclear pore Ribosome 3 Synthesis of protein Polypeptide Amino acids 28 5ʹ′ end Nitrogenous bases Pyrimidines 5ʹ′C 3ʹ′C Nucleoside Nitrogenous base Cytosine (C) Thymine (T, in DNA) Uracil (U, in RNA) Purines Phosphate group 5ʹ′C (b) Nucleotide 3ʹ′C Sugar (pentose) Adenine (A) Guanine (G) Sugars 3ʹ′ end (a) Polynucleotide, or nucleic acid Deoxyribose (in DNA) Ribose (in RNA) (c) Nucleoside components: sugars 29 5' end 3' end Sugar-phosphate backbones Base pair (joined by hydrogen bonding) Old strands Nucleotide about to be added to a new strand 3' end 5' end New strands 5' end 3' end 5' end 3' end 30 31