* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Signal Transduction
Protein folding wikipedia , lookup
Protein domain wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein structure prediction wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
Protein purification wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Western blot wikipedia , lookup
List of types of proteins wikipedia , lookup
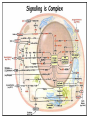
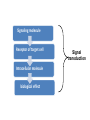
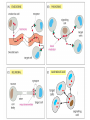
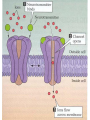
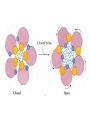
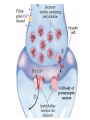
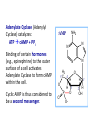
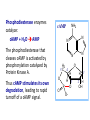
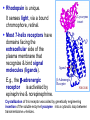
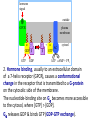
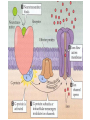
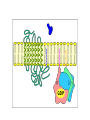
Organisms can function normally only if: All the cells of their different organs communicating effectively with their surroundings. Once a cell picks up a hormonal or sensory signal, it must transmit this information from the surface to the interior parts of the cell— Example, to the nucleus. This occurs via signal transduction pathways that are: very specific, both in their activation and in their downstream actions. Thus, the various organs in the body respond in an appropriate manner and only to relevant signals. Signal Transduction The transmission of molecular signals from a cell's exterior to its interior. Molecular signals transmitted between cells by: 1. Hormones 2. Other chemical factors 3. Sensory signals received from the environment, in form of: Light, Taste, Sound, Smell, and Touch. Upon Environment changes: Monad: responds directly Multicellular organisms: Respond with signal through a system of intercellular or intracellular communication,and consequently regulate functions of organisms. Signaling molecule Receptor of target cell Intracellular molecule biological effect Signal transduction Signaling molecules • Released by signal-producing cells, • Transfer biological signals to their target cells to initiate specific cellular responses. Two types.. • Extracellular molecules • Intracellular molecules 1. Extracellular molecules protein & peptides: Hormone, cytokine AA & its derivatives: Gly, Glu, adrenaline, thyroxine Steroid: Sex Hormone, glucocorticosteroid Fatty acid derivatives: prostaglandin (1) Paracrine signaling (local chemical mediators) • Secreted by common cells. • Reach neighboring target cells by passive diffusion. • Time of action is short. • Such as GF (2) Endocrine signal • • • • Secreted by endocrine cells. Reach target cells by blood circulation. Time of action is long. Such as insulin, thyroxine, adrenalin (3) Synaptic signal (neurotransmitters) • Secreted by neuronal cells. • Reach another neuron by synaptic gap. • Time of action is short. • Such as Acetylcholine (Ach), noradrenaline (4) Gaseous signal • Simple structure, half life is short and active in chemistry . • Such as NO, CO. GAS MOLECULE (5) Autocrine signal • Act back to their own cells. • Such as GF, cytokine, interferon, interleukin. 2. Intracellular molecule • Ca2+ ions • DG, ceramide • IP3 lipid derivatives carbohydrate derivatives • cAMP cGMP nucleotides • Ras, JAK, Raf proteins Second messenger: Small molecules synthesized in cells in response to an external signal, responsible for intracellular signal transduction. Such as Ca2+, DG, Cer, IP3, cAMP, cGMP Third messengers: The molecules which transmit message from outside to inside of nucleous or from inside to outside of nucleous, also called DNA binding protein. Proteins and peptides: Effect by membrane receptors Hormones, cytokines Amino acid derivatives: Catecholamines Fatty acid derivatives: Extracellular molecules Prostaglandins Effect by intracellular receptors Signal molecules Intracellular molecules Steroid hormones, Thyroxine, VD3 cAMP, cGMP, IP3, DG, Ca2+ 2. Receptors Receptors Specific membrane proteins, able to recognize and bind to corresponding ligand molecules, become activated, and transduce signal to next signaling molecules. Glycoprotein or Lipoprotein ligand A small molecule that binds specifically to a larger one; Example: A hormone is the ligand for its specific protein receptor. • Membrane receptors membrane Glycoprotein • Intracellular receptors Cytosol or nuclei DNA binding protein 1. membrane receptors (1) Ligand-gate ion channels type (cyclic receptor) ligand→receptor→ion channel open or close Properties of binding of H and R • highly specificity • highly affinity • saturation • reversible binding • special function model Control of receptor activity • Phosphorylation or dephosphorylation of R • Phospholipid of membrane • Enzyme catalyzed hydrolysis • G protein regulation Function of receptor (1) Recognize the special ligand (2) Binding to special ligand (3) Signal transduction biological effect Pathway of Signal Transduction serine (Ser) threonine (Thr) H H H3N+ C COO H3N+ C COO CH2 CH OH OH CH3 Many enzymes are regulated by covalent attachment of phosphate, in ester linkage, to the side-chain hydroxyl group of a particular amino acid residue (serine, threonine, or tyrosine). O Protein Kinase OH + ATP Protein Protein O P O + ADP O Pi H2O Protein Phosphatase A protein kinase transfers the terminal phosphate of ATP to a hydroxyl group on a protein. A protein phosphatase catalyzes removal of the Pi by hydrolysis. Phosphorylation may directly alter activity of an enzyme, e.g., by promoting a conformational change. Alternatively, altered activity may result from binding another protein that specifically recognizes a phosphorylated domain. E.g., 14-3-3 proteins bind to domains that include phosphorylated Ser or Thr in the sequence RXXX[pS/pT]XP, where X can be different amino acids. Binding to 14-3-3 is a mechanism by which some proteins (e.g., transcription factors) may be retained in the cytosol, & prevented from entering the nucleus. O Protein Kinase OH + ATP Protein Protein O P O + ADP O Pi H2O Protein Phosphatase Protein kinases and phosphatases are themselves regulated by complex signal cascades. For example: Some protein kinases are activated by Ca++calmodulin. Protein Kinase A is activated by cyclic-AMP (cAMP). Adenylate Cyclase (Adenylyl Cyclase) catalyzes: ATP cAMP + PPi Binding of certain hormones (e.g., epinephrine) to the outer surface of a cell activates Adenylate Cyclase to form cAMP within the cell. Cyclic AMP is thus considered to be a second messenger. NH2 cAMP N N N N H2 5' C 4' O O O H H 3' O P O- H 1' 2' H OH Phosphodiesterase enzymes catalyze: cAMP + H2O AMP N N The phosphodiesterase that cleaves cAMP is activated by phosphorylation catalyzed by Protein Kinase A. N N H2 5' C 4' O Thus cAMP stimulates its own degradation, leading to rapid turnoff of a cAMP signal. NH2 cAMP O O H H 3' P O O- H 1' 2' H OH Protein Kinase A (cAMP-Dependent Protein Kinase) transfers Pi from ATP to OH of a Ser or Thr in a particular 5-amino acid sequence. Protein Kinase A in the resting state is a complex of: • 2 catalytic subunits (C) • 2 regulatory subunits (R). R2C2 R2C2 Each regulatory subunit (R) of Protein Kinase A contains a pseudosubstrate sequence, like the substrate domain of a target protein but with Ala substituting for the Ser/Thr. The pseudosubstrate domain of (R), which lacks a hydroxyl that can be phosphorylated, binds to the active site of (C), blocking its activity. R2C2 + 4 cAMP R2cAMP4 + 2 C When each (R) binds 2 cAMP, a conformational change causes (R) to release (C). The catalytic subunits can then catalyze phosphorylation of Ser or Thr on target proteins. PKIs, Protein Kinase Inhibitors, modulate activity of the catalytic subunits (C). Pathway of G protein linked receptor H R G protein Es secondary messeger Protein kinase Phophorylation of Es or functional protein Biological effect G Protein Signal Cascade Most signal molecules targeted to a cell bind at the cell surface to receptors embedded in the plasma membrane. Only signal molecules able to cross the plasma membrane (e.g., steroid hormones) interact with intracellular receptors. A large family of cell surface receptors have a common structural motif, 7 transmembrane a-helices. Rhodopsin was the first of these to have its 7-helix structure confirmed by Rhodopsin X-ray crystallography. PDB 1F88 Rhodopsin is unique. Lysozyme insert It senses light, via a bound chromophore, retinal. Most 7-helix receptors have domains facing the extracellular side of the plasma membrane that recognize & bind signal molecules (ligands). E.g., the b-adrenergic receptor is activated by epinephrine & norepinephrine. ligand b-Adrenergic Receptor PDB 2RH1 Crystallization of this receptor was aided by genetically engineering insertion of the soluble enzyme lysozyme into a cytosolic loop between transmembrane a-helices. The signal is usually passed from a 7-helix receptor to an intracellular G-protein. Seven-helix receptors are thus called GPCR, or G-Protein-Coupled Receptors. Approx. 800 different GPCRs are encoded in the human genome. G-protein-Coupled Receptors may dimerize or form oligomeric complexes within the membrane. Ligand binding may promote oligomerization, which may in turn affect activity of the receptor. Various GPCR-interacting proteins (GIPs) modulate receptor function. Effects of GIPs may include: altered ligand affinity receptor dimerization or oligomerization control of receptor localization, including transfer to or removal from the plasma membrane promoting close association with other signal proteins G-proteins are heterotrimeric, with 3 subunits a, b, g. A G-protein that activates cyclic-AMP formation within a cell is called a stimulatory G-protein, designated Gs with alpha subunit Gsa. Gs is activated, e.g., by receptors for the hormones epinephrine and glucagon. The b-adrenergic receptor is the GPCR for epinephrine. hormone signal outside GPCR The a subunit of a G-protein (Ga) binds GTP, & can hydrolyze it to GDP + Pi. plasma membrane agga AC GDP bbGTP GTP GDP cytosol ATP cAMP + PPi a & g subunits have covalently attached lipid anchors that bind a G-protein to the plasma membrane cytosolic surface. Adenylate Cyclase (AC) is a transmembrane protein, with cytosolic domains forming the catalytic site. hormone signal outside GPCR plasma membrane agga AC GDP bbGTP GTP GDP cytosol ATP cAMP + PPi The sequence of events by which a hormone activates cAMP signaling: 1. Initially Ga has bound GDP, and a,b, & g subunits are complexed together. Gb,g, the complex of b & g subunits, inhibits Ga. hormone signal outside GPCR plasma membrane agga AC GDP bbGTP GTP GDP cytosol ATP cAMP + PPi 2. Hormone binding, usually to an extracellular domain of a 7-helix receptor (GPCR), causes a conformational change in the receptor that is transmitted to a G-protein on the cytosolic side of the membrane. The nucleotide-binding site on Ga becomes more accessible to the cytosol, where [GTP] > [GDP]. Ga releases GDP & binds GTP (GDP-GTP exchange). hormone signal outside GPCR plasma membrane agga AC GDP bbGTP GTP GDP cytosol ATP cAMP + PPi 3. Substitution of GTP for GDP causes another conformational change in Ga. Ga-GTP dissociates from the inhibitory bg complex & can now bind to and activate Adenylate Cyclase. hormone signal outside GPCR plasma membrane agga AC GDP bbGTP GTP GDP cytosol ATP cAMP + PPi 4. Adenylate Cyclase, activated by the stimulatory Ga-GTP, catalyzes synthesis of cAMP. 5. Protein Kinase A (cAMP Dependent Protein Kinase) catalyzes transfer of phosphate from ATP to serine or threonine residues of various cellular proteins, altering their activity. Turn off of the signal: 1. Ga hydrolyzes GTP to GDP + Pi. (GTPase). The presence of GDP on Ga causes it to rebind to the inhibitory bg complex. Adenylate Cyclase is no longer activated. 2. Phosphodiesterases catalyze hydrolysis of cAMP AMP. 3. Protein Phosphatase catalyzes removal by hydrolysis of phosphates that were attached to proteins via Protein Kinase A. Signal amplification is an important feature of signal cascades: One hormone molecule can lead to formation of many cAMP molecules. Each catalytic subunit of Protein Kinase A catalyzes phosphorylation of many proteins during the lifetime of the cAMP. Different isoforms of Ga have different signal roles. E.g.: • The stimulatory Gsa, when it binds GTP, activates Adenylate cyclase. • An inhibitory Gia, when it binds GTP, inhibits Adenylate cyclase. Different effectors & their receptors induce Gia to exchange GDP for GTP than those that activate Gsa. The complex of Gb,g that is released when Ga binds GTP is itself an effector that binds to and activates or inhibits several other proteins. E.g., Gb,g inhibits one of several isoforms of Adenylate Cyclase, contributing to rapid signal turnoff in cells that express that enzyme. Cholera toxin catalyzes covalent modification of Gsa. • ADP-ribose is transferred from NAD+ to an arginine residue at the GTPase active site of Gsa. • ADP-ribosylation prevents GTP hydrolysis by Gsa . • The stimulatory G-protein is permanently activated. Pertussis toxin (whooping cough disease) catalyzes ADPribosylation at a cysteine residue of the inhibitory Gia, making it incapable of exchanging GDP for GTP. • The inhibitory pathway is blocked. ADP-ribosylation is a general mechanism by which activity of many proteins is regulated, in eukaryotes (including mammals) as well as in prokaryotes.