* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 1 Matter and Change
Electrolysis of water wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemical warfare wikipedia , lookup
Water splitting wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Fluorochemical industry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Photopolymer wikipedia , lookup
Organic chemistry wikipedia , lookup
Water pollution wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Condensed matter physics wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Chemical element wikipedia , lookup
Gas chromatography wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical industry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Atomic theory wikipedia , lookup
Drug discovery wikipedia , lookup
History of chemistry wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
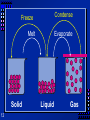
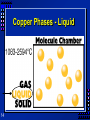
Chapter 1 “Matter and Change” 1 Section 1.3 Properties of Matter OBJECTIVES: –Define physical property, and list several common physical properties of substances. –Distinguish between matter and substances 2 Section 1.4 Properties of Matter OBJECTIVES: –Differentiate among three states of matter. 3 Section 1.5 Properties of Matter OBJECTIVES: –Describe a physical change. 4 Matter Matter is anything that: a) has mass, and b) takes up space Mass = a measure of the amount of “stuff” (or material) the object contains (don’t confuse this with weight, a measure of gravity) Volume = a measure of the space occupied by the object 5 Substances Matter that has a uniform and definite composition. Either a pure element or a compund – Salt is a substance » Every sample of NaCl tastes the same, melts at the same temp., and is 39.3% Na and 60.7% Cl by mass. – What about a cup of coffee? » No, each cup can have different strengths or different amounts of sugar. 6 Properties are… Words that describe matter (adjectives) Physical Properties- a property that can be observed and measured without changing the material’s composition. Examples- color, hardness, m.p., b.p. Chemical Properties- a property that can only be observed by changing the composition of the material. Examples- ability to burn, decompose, ferment, react with, etc. 7 States of matter 1) Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. – Vapor- a substance that is currently a gas, but normally is a liquid or solid at room temperature. (Which is correct: “water gas”, or “water vapor”?) 8 States of Matter Definite Definite Volume? Shape? Solid Liquid Gas 9 YES YES NO Result of a TemperatureI Will it Compress? ncrease? YES Small Expans. NO NO Small Expans. NO NO Large Expans. YES 4th state: Plasma - formed at high temperatures; ionized phase of matter as found in the sun 10 Three Main Phases 11 Condense Freeze Evaporate Melt Solid 12 Liquid Gas Copper Phases - Solid 13 Copper Phases - Liquid 14 Copper Phases – Vapor (gas) 15 Physical vs. Chemical Change Physical change will change the visible appearance, without changing the composition of the material. – Boil, melt, cut, bend, split, crack – Is boiled water still water? Can be reversible, or irreversible Chemical change - a change where a new form of matter is formed. – Rust, burn, decompose, ferment 16 Recap What makes matter a substance? – Has a uniform and definite composition What is the difference between a solid, liquid and gas? – Solid has a definite shape – Liquid takes the shape of it’s container – Gas will expand to volume it occupies What are some examples of physical changes? – Melting, Bending, Cutting apart, freezing, dissolving 17 Section 1.6 Mixtures OBJECTIVES: –Categorize a sample of matter as a substance or a mixture. 18 Section 1.6 Mixtures OBJECTIVES: –Distinguish between homogeneous and heterogeneous samples of matter. 19 Section 1.6 Mixtures OBJECTIVES: –Describe two ways that components of mixtures can be separated. 20 Mixtures are a physical blend of at least two substances; have variable composition. They can be either: 1) Heterogeneous – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) Homogeneous - same composition throughout; called “solutions” • Kool-aid, air, salt water Every part keeps it’s own properties. 21 Solutions are homogeneous mixtures Mixed molecule by molecule, thus too small to see the different parts Can occur between any state of matter: gas in gas; liquid in gas; gas in liquid; solid in liquid; solid in solid (alloys), etc. Thus, based on the distribution of their components, mixtures are called homogeneous or heterogeneous. 22 Mixtures two or more substances mixed together …have varying composition …have varying properties The substances are NOT chemically bonded, and they… retain their individual properties. Tea, orange juice, oceans, and air are mixtures. 23 Two Types of Mixtures homogeneous: (or solution) particles are microscopic; sample has same composition and properties throughout; evenly mixed e.g., salt water Kool Aid alloy: a homogeneous mixture of metals e.g., 24 bronze (Cu + Sn) pewter (Pb + Sn) brass (Cu + Zn) Two Types of Mixtures (cont.) heterogeneous: different composition and properties in the same sample; unevenly mixed tossed salad e.g., raisin bran suspension: settles over time e.g., 25 paint snowy-bulb gifts Contrast… 24K GOLD 24/24 atoms are gold 14/24 atoms are gold pure gold mixture of gold & copper element homogeneous mixture Au + Cu Au 26 14K GOLD Phase? The term “phase” is used to describe any part of a sample with uniform composition of properties. A homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases. Note Figure 1.8, page 12 27 Separating Mixtures Some can be separated easily by physical means: rocks and marbles, iron filings and sulfur (use magnet) Differences in physical properties can be used to separate mixtures. Filtration - separates a solid from the liquid in a heterogeneous mixture (by size) 28 Separation of a Mixture Components of dyes such as ink may be separated by paper chromatography. 29 Separation of a Mixture Distillation: takes advantage of different boiling points. NaCl boils at 1415 oC 30 Separating Mixtures (cont.) 5. density: “sink vs. float” perhaps use a centrifuge blood after highspeed centrifuging decant: to pour off the liquid 31 Section 1.7 Elements and Compounds OBJECTIVES: –Explain the differences between an element and a compound. 32 Section 1.7 Elements and Compounds OBJECTIVES: –Distinguish between a substance and a mixture. 33 Section 1.8 Elements and Compounds OBJECTIVES: –Identify the chemical symbols of elements, and name elements given their symbols. 34 Substances are either: a) elements, or b) compounds 35 Substances: element or compound 36 Elements- simplest kind of matter – cannot be broken down any simpler and still have properties of that element! – all one kind of atom. Compounds are substances that can be broken down only by chemical methods – when broken down, the pieces have completely different properties than the original compound. – made of two or more atoms, chemically combined (not just a physical blend!) Classifying Matter (Pure) Substances …have a fixed composition …have fixed properties 37 ELEMENTS COMPOUNDS e.g., Fe, N2, S8, U e.g., H2O, NaCl, HNO3 sulfur (S8) sodium chloride (NaCl) Pure substances have a chemical formula. Compound vs. Mixture 38 Compound Mixture Made of one kind of substance Made of more than one kind of substance Made by a chemical change Made by a physical change Definite composition Variable composition Which is it? Mixture Element Compound 39 Elements vs. Compounds Compounds can be broken down into simpler substances by chemical means, but elements cannot. A “chemical change” is a change that produces matter with a different composition than the original matter. 40 Chemical Change A change in which one or more substances are converted into different substances. Heat and light are often evidence of a chemical change. 41 Properties of Compounds Quite different properties than their component elements. Due to a CHEMICAL CHANGE, the resulting compound has new and different properties: • Table sugar – carbon, hydrogen, oxygen • Sodium chloride – sodium, chlorine • Water – hydrogen, oxygen 42 Chart for Classifying Matter MATTER PURE SUBSTANCE ELEMENT MIXTURE COMPOUND HETEROGENEOUS HOMOGENEOUS 43 Symbols & Formulas Currently, there are 117 elements Elements have a 1 or two letter symbol, and compounds have a formula. An element’s first letter always capitalized; if there is a second letter, it is written lowercase: B, Ba, C, Ca, H, He Start learning the elements names and symbols listed in Table A.1 on page 782 Some names come from Latin or other languages; note Table 1.5, page 17 44 Recap What is a mixture? – A physical blend of two or more substances What is the difference between homogeneous and heterogeneous mixtures? – Homogeneous – uniform composition – Heterogeneous – mixed/not uniform composition What is the difference between an element and a compound? – Elements: Made of one type of atom, can not be separated into simpler substances – Compound: Made of different elements. Can be separated by chemical means. What are the English names of these elements? Au, Cu, Hg, Pb, Sn, Fe, K, Na Gold, Copper, Mercury, Lead, Tin, Iron, Potassium, Sodium 45 Lab Write Ups Purpose Hypothesis Procedure & Materials Observations/Data (organized) Analysis Make conclusions Communicate 46 Lab Write Ups Purpose: Ask a question and use to write the purpose. “Can I tell if ink is a mixture using chromatography to separate it’s components?” Change to: Purpose: To use chromatography to determine if an ink is an mixture. Hypothesis: An ink is not a mixture and will not separate into different components when using chromatography. 47 Lab Write Ups 48 Procedures: List the exact steps you will use to complete your experiment. Use diagrams/photo’s when appropriate (a picture is worth a thousand words). Materials: List the materials you will use. Observe and collect data: Organize it table formate. Analyze the data: Use graphs and statistics to help with analysis. Make conclusions and communicate. Write a paragraph or two discussing your analysis and what conclusions you can make from your observations and data. (Communication comes in the form of a lab report with all of the above steps written down or a presentation with each step discussed and presented.) Lab Write Ups 49 What did you learn? Is Ink a mixture or a substance? Why? How many different components did you observe? Section 1.9 Chemical Reactions OBJECTIVES: –Describe what happens during a chemical change. 50 Section 1.9 Chemical Reactions OBJECTIVES: –Identify four possible clues that a chemical change has taken place. 51 Section 1.10 Chemical Reactions OBJECTIVES: –Apply the law of conservation of mass to chemical reactions. 52 Chemical Changes The ability of a substance to undergo a specific chemical change is called a chemical property. • iron plus oxygen forms rust, so the ability to rust is a chemical property of iron During a chemical change (also called chemical reaction), the composition of matter always changes. 53 Chemical Reactions are… When one or more substances are changed into new substances. Reactants- the stuff you start with Products- what you make The products will have NEW PROPERTIES different from the reactants you started with Arrow points from the reactants to the new products 54 Copper + Nitric Acid Demo Cu(s) + 4HNO3(aq) Red/Orange 55 Clear Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l) Blue Brown Recognizing Chemical Changes 1) Energy is absorbed or released (temperature changes hotter or colder) 2) Color changes 3) Gas production (bubbling, fizzing, or odor change; smoke) 4) formation of a precipitate - a solid that separates from solution (won’t dissolve) 5) Irreversibility - not easily reversed But, there are examples of these that are not chemical – boiling water bubbles, etc. 56 Conservation of Mass During any chemical reaction, the mass of the products is always equal to the mass of the reactants. All the mass can be accounted for: –Burning of wood results in products that appear to have less mass as ashes; where is the rest? Law 57 of conservation of mass - Page 55 43.43 g Original mass = 43.43 g Final mass reactants 58 = product Recap 59 What are some examples of evidence of a chemical reaction? – Color, bubbles, gas, temperature, light, flame, precipitates, irreversible What is the difference between a chemical and physical change? – Chemical: a new substance is produced – Physical: no new substance is created What does the law of conservation of mass mean? – The mass of the products is always equal to the mass of the reactants. 60 Chapter 1 HW HW Problems: (34 pts) 38, 39, 40, 41, 42, 46, 47, 57, 61 61 Due Monday 9/15 Chapter 1 HW Review Problem 38: Which are heterogeneous or homogeneous? A: spaghetti sauce > heterogeneous B: glass > homogeneous C: table sugar > homogeneous D: river water > heterogeneous E: cough syrup > homogeneous F. Nitrogen > homogeneous 64 Chapter 1 HW Review 65 Problems 39: How to distinguish between and element and compound? Compounds can be chemically separated Elements can not be separated into simpler substances chemically Problem 40: Element, Compound or Mixture? A: spaghetti sauce > mixture B: glass > mixture C: table sugar > compound D: river water > mixture E: cough syrup > mixture F. Nitrogen > element Problem 41: The chemical symbols are: A: copper > Cu B: oxygen > O C: phosphorus > P D: silver > Ag E: sodium >Na F. helium > He Chapter 1 HW Review 66 Problems 42: Name the elements in each compound A. NH4Cl: nitrogen, hydrogen, chlorine B. KMnO4: potassium, manganese, oxygen C. C3H7OH: carbon, hydrogen, oxygen D. CaI2: calcium, iodine Problem 46: Why is mass not lost when burning a candle? Some of the products (CO2 & H2O) are in gas form and go into the surrounding air. Problem 47: When 40g of NH4NO3 explode and 14g of N2 and 8g of O2 are formed, how many grams of H2O are formed? 40g – 14g – 8g = 18 grams of H2O Chapter 1 HW Review 67 Problems 57: Silicon properties A. Blue-gray > physical B. Brittle > physical C. Insoluble in water > physical D. Melts at 1410 C > physical E. Reacts with fluorine (usually HF) > chemical Problem 61: What is the relationship between particles in different states of matter? Solid: Tightly packed and linked Liquid: Touching/In contact and loosely linked Gas: Far apart, not linked at all Chapter 1 Review “Matter and Change” Chemistry Clatskanie High School 69 Chapter 1 Review What is another name for homogeneous mixtures? Solutions The chemical formula of a compound does NOT indicate the Physical properti A substance that forms a vapor is generally in what physical state at room temperature? Solid or Liquid 70 Chapter 1 Review Which state of matter has a definite volume and takes the shape of its container? Liquid The first figure in a properly written chemical symbol always is Capitalized ______ 71 Chapter 1 Review What is one difference between a mixture and a compound? –A mixture can be separated by physical means. What is true about compounds: a) they have compositions that vary, or b) they are substances? b) substances Which of the following is a homogeneous mixture: –a) salt water, or b) beef stew? 72 Chapter 1 Review Which action changes the identity of the substance: a) breaking an ice cube, or b) corroding iron? Which of the following CANNOT be classified as a substance: a) air, or b) table salt? What must occur for a change to be a chemical reaction? –A new substance is formed 73 Chapter 1 Review What distinguishes a substance from a mixture? –A substance has a uniform and definite composition Separating a solid from a liquid by evaporating the liquid is called. –Drying or distillation Which of the following is a chemical property: a) freezing point, or b) ability to react with oxygen? 74 Chapter 1 Review Which of the following is a chemical property of water at 4 oC: a) its color, or b) the ability to decompose into hydrogen and oxygen? Which state of matter takes both the shape and volume of its container? –Gas 75 Chapter 1 Review When paper turns yellow-brown upon exposure to sunlight, what type of change is likely taking place? –Chemical Which of the following items is NOT a compound: a) salad dressing, or b) table salt? Which of the following is NOT an example of matter: a) heat, or b) air? 76 Chapter 1 Review Which of the following materials is a substance: a) gasoline, or b) silver? A chemical change occurs when a piece of wood: a) is split, or b) decays? What is the chemical symbol for iron? –Fe Which of the following is a physical change: a) rotting of food, or b) evaporation? 77 Chapter 1 Review What do chemical symbols and formulas represent, respectively? –Elements and compounds Which of the following does NOT involve a physical change: a) mixing, or b) decomposing? Which of the following is NOT a physical property of water: a) it is a colorless liquid, or b) it is composed of hydrogen and oxygen? 78 Chapter 1 Review Which substance has a chemical symbol that is derived from a Latin name: a) calcium, or b) potassium? Know some examples of physical properties of matter. –Color, BP, MP, density, conductivity, odor Which of the following does NOT indicate that a chemical change may have taken place: a) gas production, or 79 b) tearing or bending? Chapter 1 Review A golf ball has more mass than a tennis ball because it –has higher density (more matter) Which of the following is a heterogeneous mixture: a) soil, or b) salt water? Which of the following is a heterogeneous mixture: a) vinegar in water, or b) oil and vinegar? 80 Chapter 1 Review Which of the following is true for all chemical reactions: a) the total mass of reactants = total mass of products, or b) the total mass of the reactants increases? Which state of matter has a definite shape and a definite volume? –Solid 81 Chapter 1 Review What happens to matter during a chemical reaction: a) some matter is destroyed, or b) matter is neither created nor destroyed? Which of the following are considered physical properties of a substance: a) color and odor, or b) melting and boiling points? 82 Chapter 1 Review Which of the following is NOT a physical change: a) melting cheese, or b) fermenting of cheese? An example of a homogeneous mixture is: a) stainless steel, or b) noodle soup? Which of the following is NOT a physical change to a metal: a) melting, or b) rusting? 83 Chapter 1 Review Which of the following indicates that a chemical change has happened during cooking: a) the food darkens, or b) bubbles form in boiling water? Which of the following is used for chemical symbols today: a) letters, or b) drawings? When an iron nail is ground into powder, its mass ____. –Stays the same 84 Chapter 1 Review Which of the following processes does NOT involve a change in chemical properties: a) boiling, or b) burning? A substance that can be separated into two or more substances only by a chemical change is a(n): a) mixture, or b) compound? 85 Chapter 1 Review How many grams of liquid water are produced when 60 grams of ice melt? –60 grams In how many physical states does water commonly exist? –Three What is the melting point of water in degrees Celsius? –0 degrees C 86 Chapter 1 Review What is the boiling point of water in degrees Celsius? –100 degrees C 87