* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download excited states
Molecular Hamiltonian wikipedia , lookup
Photosynthesis wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Double-slit experiment wikipedia , lookup
Atomic theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
Electron configuration wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Chemical imaging wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Electron scattering wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Nitrogen-vacancy center wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Fluorescence correlation spectroscopy wikipedia , lookup
Population inversion wikipedia , lookup
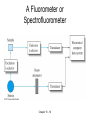
CHAPTER 15: MOLECULAR LUMINESCENCE LUMINESCENCE TECHNIQUES • Emission of light is used to determine certain properties,e e.g.structure and concentration, of the emitting species. • Deactivation processes involved in converting a substance from excited state to ground state: • the emission of heat, • activation of a chemical reaction or • emission of radiation of the same or a modified wavelength. • Forms of photoluminescence (luminescence after absorption) are fluorescence (short lifetime) and phosphorescence (long lifetime). • Approximately 10x more sensitive than absorption techniques:ppb detection limit • Limited number of systems that photoluminesce. • Luminescence observed for simple and complex systems and for all three phases. Chapter 15 - 2 Theory Atoms: e.g. dilute Na (g) the • 3s 3p transition occurs by absorption at l = 5895 and 5890 A. with a lifetime 10-8 sec, • the electron returns to the ground state isotropically (isotropically) emitting hn with the wavelength of emission being the same as the wavelength of excitation. resonance fluorescence. • Polyatomic Systems – Resonance fluorescence observed – Emission of radiation of longer l (called a Stoke's shift) more common.. Most fluorescent systems are complex organic compounds with 1 or more aromatic functional groups so that the commonly observed transitions are: n. Chapter 15 - 3 EXCITED STATES • • • • • Multiplicity (number of lines observed when the molecule is placed in a magnetic field) is related number of unpaired spins in the molecule (S):. M = 2•S + 1. Most molecules have an even number of electrons which means that all of their electrons in the ground state must be paired: Singlet state (M = 2•0 + 1);. all electrons in ground state paired. Doublet state : (M = 2•½ + 1 = 2)a free radical (substance that has an odd number of electrons);. electrons can have 2 orientations in the magnetic field-opposed to the field and aligned. Triplet state: (M = 2•1 + 1 = 3), excited state in which excited electron spin is flipped so that the spins are parallel. Singlet state Triplet state Diamagnetic Paramagnetic Probable Less probable -8 -13 Lifetime 10 -10 sec Lifetime up to ≥1 s Chapter 15 - 4 ENERGY LEVEL DIAGRAM • Let ground , the first excited, second excited etc. electronic states be: S0, S1, S2 and etc..for all of the possible singlet states. • Triplet states would then be T1, T2 and so on . • All electronic state has several vibrational and rotational states. Chapter 15 - 5 Decay Processes • Internal conversion Movement of electron from one electronic state to another without emission of a photon, e.g. S2 S1) lasts about 10-12 sec. • Predissociation internal conversion electron relaxes into a state where energy of that state is high enough to rupture the bond. • Vibrational relaxation (10-10-10-11sec)- Energy loss associated with electron movement to lower vibrational state without photon emission. • Intersystem crossing: Conversion from singlet state to a triplet state. e.g. S1 to T1 • External conversion is a non-radiative process in which energy of an excited state is given to another molecule (e.g. solvent or other solute molecules). Related to the collisional frequency of excited species with other molecules in the solution. Cooling the solution minimizes this effect. Chapter 15 - 6 QUANTUM YIELD • Only a fraction of the photon absorbed result in fluorescence. Fraction called Quantum yield (efficiency), F: F • • # emitted # absorbed Excite molecule in say the S1 state can undergo a transition back to the ground state: S1 S0 + hn. The emitted photon is the useful fluorescence line and takes about 10-6-10-10 sec to occur. Rate of all processes which involved the absorption or emission of a photon can be written in terms of a first order rate equation: Instrumental Analysis,Christian and O’Reilly, p. 251 Chapter 15 - 7 KINETICS OF ADSORPTION • • • • • • • • The intensity of the light absorbed is .DP = Po - PT = rate of absorption where Po = photon flux to sample, PT = photon flux out of sample. At steady state rate of absorption equals rate of fluorescence or: DP = (kIC + kISC + kf + kQ[Q])[S1] where kQ = rate constant for quenching process-a second order process since the [Q] is also important in determining the relative rate of this process. Let [S1] = steady state concentration of S1 molecules. The rate of fluorescence Pf = FDP = F(kIC + kISC + kf + kQ[Q])[S1] and Pf = kf[S1]. kf Combine and rearrange: F = k IC + k ISC + k f + k q [Q] 1 large F means a large kf. The lifetime of the fluorescing state is given by f kf The inverse relationship between the rate constant and the lifetime tells us that a process having a large rate constant has a short lifetime and will have the largest fluorescence intensity. Chapter 15 - 8 FLUORESCENCE INTENSITY VS CONCENTRATION • Before fluorescence occurs absorption must occur. The absorption process given by Beer's Law: - l og • • • • P Po = - log T = A = e b C where e= k/2.303 = molar absorptivity. We will use this in the development of the fundamental equation for fluorescence. Earlier we stated that Pf = rate of fluorescence = F(kIC + kISC + kf + kQ[Q])[S1] = FDP = F[Po - PT]. Beers law can be written as: PT = Po×10-ebC = Po×e-2.303ebC. Substituting into the fluorescence equation gives: Pf = F[Po - Po×e-2.303ebC] = FPo[1 - e-2.303ebC]. Chapter 15 - 9 Concentration Dependence 2 • From the mathematics handbook: x2 x3 xn + + + 2! 3! n! We substitute this for the exponential terms to get the following: ex = 1 + x + • (-2.303 e b C ) 2 (-2.303 e b C ) 3 Pf Po F 1 - 1 + 2.303 e b C 2! 3! (-2.303 e b C )2 (-2.303 e b C )3 Po F 2.303 e b C + 2! 3! For dilute solutions A = ebC is small which will make the squared and higher powered terms quite small. e.g. if A = 0.05, then the second term is while the first term is 2.303×0.05 = 0.115. • The fluorescence equation then reduces to: • Pf = Po×F×2.303×e×b×C or Pf = K×C or a linear response in the fluorescence intensity with concentration will be observed as long as the A < 0.05 • Chapter 15 - 10 Fluorescence Intensity vs Conc. • When performing a fluorometric analysis, Pf is measured independently of Po so that it is not necessary to determine P0 i.e. only one measurement of intensity is made. • Remember that Beer’s law for absorption requires measurement of both P and P0 • In the fluorescence experiment, one can increase Po and should expect an increase in Pf. Thus, one can increase the sensitivity to the analyte by increasing the power of the exciting light. • In the absorption experiment, one needs the ratio of the input and output power. Increasing the input power also increases the output power but does little to the ratio. • This makes fluorescent techniques inherently more sensitive than absorption techniques. The detection limits are 10-8M; in fluorimetry they are 10-12M. Chapter 15 - 11 F AND TRANSITION TYPE • • • • • • • • • • • • In absorption spectrum of organics we can observe the following transitions to excited states: ;n ;;n Fluorescence: .Same orbitals possible. ., and n transitions are seldom observed however when the l of the transition is 240 nm(UV) since the energy of the transition is often high enough to dissociate the molecule. Less energetic transitions ; n transitions observed. .F, for the transition is usually greatest since process has shortest average lifetime and greatest molar absorptivity. Other affects on the quantum yield: In our equation describing quantum yield: kf .F = kIC + kISC + kF + k q [Q] All terms except kf must be minimized to obtain a large fluorescent signal. Chemical structures that minimize one or more of the these rate constants increase the quantum yield. Structural rigidity: Decreases the chances of vibrational and rotational de-excitation (which we have called IC (internal conversion).. Prevents the loss of energy by the internal conversion process so that the fluorescent yield is higher in rigid molecules. Ring structures with alternating single and double bonds (conjugation) that are aromatic usually best fluorescing compounds, although highly conjugated aliphatic compounds may also fluoresce. Temperature: Raising the temperature of a system increases the collisional frequency between excited molecules and the solvent which increases the amount of external conversion. Solvent: Decrease in solvent viscosity also leads to increase in external conversion and a decrease in fluorescence intensity. Chapter 15 - 12 PHOTOLUMINESCENT ANALYSIS • Since many compounds fluoresce at the same l, fluorescence cannot be used for qualitative analysis. • Quantitative analysis of a large number of organic compounds in particular polycyclic molecules with extensive conjugation possible. E.g. Vitamin A which has a blue-green fluorescence with lmax » 500 nm in ETOH. • Often molecules will be polynuclear aromatics such as phenol. • Inorganic species – Direct: form a fluorescent complex with organic species and measure the fluorescent intensity. Fluorometric agents usually polyfunctional group aromatic compounds. – Indirect: diminution of fluorescence measured when the ion is added to a fluorescent solution. Reaction stoichiometry between the ion and the fluorescent reagent must be known. Chapter 15 - 13 Fluorescence Problem 5.00 mL of an unknown zinc solution was placed in each of two separatory funnels and 4.00 mL of 1.10 ppm Zn2+ was added to the second solution. Each was extracted with three 5 mL aliquots of CCl4 containing an excess of 8-hydroxyquinoline. The extracts were then diluted and their fluorescence measured with a fluorometer. The fluorescent intensities were 6.12 for the solution containing no added zinc and 11.16 for the other solution. Determine the concentration of the original zinc solution. • Strategy: – Determine concentration of final solution containing the unknown (Standard Addition Method). – Determine concentration of the original solution. Chapter 15 - 14 INSTRUMENTATION • Source: Hg or Xe arc lamp is used. (continuous radiation in the 250600 nm range is produced). • Monochromators or filters: needed to select both wavelength of excitation emission. • Monochromators are used when dealing with narrow absorption or emission peaks while filters may be used when peaks are not as narrow. • When filters are used, one is limited to wavelength range that passes through the particular filter used. • Instruments using filters are called fluorometers while instrument using monochromators are called spectrofluorimeter. • Cells and Cell compartments: cylindrical or rectangular (less scattering rectangular cell); quartz or glass depending upon the wavelength range needed; Outlet of the sample cell usually 90° from the inlet.(minimizes source light at detector. • Detector: Phototube or photomultiplier (small signals) Chapter 15 - 15 A Fluorometer or Spectrofluorometer Chapter 15 - 16 A Sectrofluorometer Chapter 15 - 17