Chem*3560 Lecture 6: Allosteric regulation of enzymes
... When ATP and CTP are both present, they compete for the same binding site (see below), and the relative concentration of the two nucleotides will determine whether a positive or negative effect is observed. CTP has slightly more affinity, so [ATP] has to be significantly higher than [CTP] for a posi ...
... When ATP and CTP are both present, they compete for the same binding site (see below), and the relative concentration of the two nucleotides will determine whether a positive or negative effect is observed. CTP has slightly more affinity, so [ATP] has to be significantly higher than [CTP] for a posi ...
Book Problems Chapter 2
... (a) ATP + H2O → ADP + Pi The transporter must include a cytosolic nucleotide binding site that changes its conformation when its bound ATP is hydrolyzed to ADP. This conformational change must be communicated to the membrane-spanning portion of the protein, where the transported substrate binds. (b) ...
... (a) ATP + H2O → ADP + Pi The transporter must include a cytosolic nucleotide binding site that changes its conformation when its bound ATP is hydrolyzed to ADP. This conformational change must be communicated to the membrane-spanning portion of the protein, where the transported substrate binds. (b) ...
Lactic Acid System - PhysicalEducationatMSC
... When insufficient oxygen is available to breakdown the pyruvate then lactate is produced Lactate enters the surrounding muscle cells, tissue and blood The muscle cells and tissues receiving the lactate either breakdown the lactate to fuel (ATP) for immediate use or use it in the creation of glycogen ...
... When insufficient oxygen is available to breakdown the pyruvate then lactate is produced Lactate enters the surrounding muscle cells, tissue and blood The muscle cells and tissues receiving the lactate either breakdown the lactate to fuel (ATP) for immediate use or use it in the creation of glycogen ...
1 - Humble ISD
... 90. The link reaction produces Acetyl CoA (2C) from the input substrate (usually pyruvate). The extra carbon is released as carbon dioxide. Acetyl CoA can also be produced from fatty acids. When the fatty acid chain contains an even number of carbons, no CO2 is released. How many Acetyl CoA molecule ...
... 90. The link reaction produces Acetyl CoA (2C) from the input substrate (usually pyruvate). The extra carbon is released as carbon dioxide. Acetyl CoA can also be produced from fatty acids. When the fatty acid chain contains an even number of carbons, no CO2 is released. How many Acetyl CoA molecule ...
Biology
... There are two kinds of nucleic acids, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). RNA contains the sugar ribose. DNA contains the sugar deoxyribose. ...
... There are two kinds of nucleic acids, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). RNA contains the sugar ribose. DNA contains the sugar deoxyribose. ...
Nucleic Acid metabolism De Novo Synthesis of Purine
... phase of control is involved with maintaining an appropriate balance (not equality) between ATP and GTP. Each one stimulates the synthesis of the other by providing the energy. Feedback inhibition also controls the branched portion as GMP inhibits the conversion of IMP to XMP and AMP inhibits the co ...
... phase of control is involved with maintaining an appropriate balance (not equality) between ATP and GTP. Each one stimulates the synthesis of the other by providing the energy. Feedback inhibition also controls the branched portion as GMP inhibits the conversion of IMP to XMP and AMP inhibits the co ...
Photosynthesis - mleonessciencepage
... protons across the thylakoid membrane. Protons are pushed through ATP Synthase in the thylaloid membrane Adds a phosphate group to ADP making ATP ...
... protons across the thylakoid membrane. Protons are pushed through ATP Synthase in the thylaloid membrane Adds a phosphate group to ADP making ATP ...
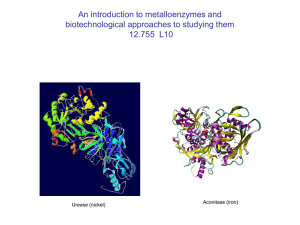
Metalloenzyme Functions
... Key enzyme in the nitrification reaction: ammonia (NH3) hydroxylamine (NH2OH) nitrite (NO2-) ...
... Key enzyme in the nitrification reaction: ammonia (NH3) hydroxylamine (NH2OH) nitrite (NO2-) ...
video slide - Biology at Mott
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
Tricarboxylic acid cycle (TCA cycle, also called the Krebs cycle or
... amino acids, and fatty acids converge, their carbon skeletons being converted to CO2 and H2O. This oxidation provides energy for the production of the majority of ATP in most animals, including humans. The cycle occurs totally in the mitochondria. Intermediates of the TCA cycle can also be synthesiz ...
... amino acids, and fatty acids converge, their carbon skeletons being converted to CO2 and H2O. This oxidation provides energy for the production of the majority of ATP in most animals, including humans. The cycle occurs totally in the mitochondria. Intermediates of the TCA cycle can also be synthesiz ...
Reactions of the citric acid cycle
... amino acids, and fatty acids converge, their carbon skeletons being converted to CO2 and H2O. This oxidation provides energy for the production of the majority of ATP in most animals, including humans. The cycle occurs totally in the mitochondria. Intermediates of the TCA cycle can also be synthesiz ...
... amino acids, and fatty acids converge, their carbon skeletons being converted to CO2 and H2O. This oxidation provides energy for the production of the majority of ATP in most animals, including humans. The cycle occurs totally in the mitochondria. Intermediates of the TCA cycle can also be synthesiz ...
GRADE 11F: Biology 1
... They know that ATP is the immediate energy source in cellular processes and relate this to respiration. They outline the reaction steps in the glycolysis, the Krebs cycle and oxidative phosphorylation stages of respiration. Students who progress further understand the basic biochemistry of anaerobic ...
... They know that ATP is the immediate energy source in cellular processes and relate this to respiration. They outline the reaction steps in the glycolysis, the Krebs cycle and oxidative phosphorylation stages of respiration. Students who progress further understand the basic biochemistry of anaerobic ...
Welcome to Class 14 - (canvas.brown.edu).
... Through the reactions of nitrification and denitrification, eventually, all nitrogen in the biosphere would be converted to N2, if it were not for:! ...
... Through the reactions of nitrification and denitrification, eventually, all nitrogen in the biosphere would be converted to N2, if it were not for:! ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)