normal myocardial metabolism: fueling cardiac contraction
... (Krebs) cycle. Regulation of PDH by fatty acids, for example, limits glucose entry into the Krebs cycle and is a critical step regulating myocardial substrate choice and utilization.19,20 Lactate decarboxylation is another important source of pyruvate for PDH, because lactate produced by other organ ...
... (Krebs) cycle. Regulation of PDH by fatty acids, for example, limits glucose entry into the Krebs cycle and is a critical step regulating myocardial substrate choice and utilization.19,20 Lactate decarboxylation is another important source of pyruvate for PDH, because lactate produced by other organ ...
glyoxylate cycle
... Gluconeogenesis and starch/glycogen synthesis In animals, lactate formed anaerobically in muscles is converted to glucose in liver and kidney and stored as glycogen or released as blood glucose. In plants, G3P product of photosynthesis is converted to starch and stored in chloroplasts or conver ...
... Gluconeogenesis and starch/glycogen synthesis In animals, lactate formed anaerobically in muscles is converted to glucose in liver and kidney and stored as glycogen or released as blood glucose. In plants, G3P product of photosynthesis is converted to starch and stored in chloroplasts or conver ...
Enzyme cofactors
... (de)hydrogenaion of the C—O or C—N bond proton released to the solution example: lactate dehydrogenase (lactate + NAD+ → → pyruvate + NADH +H+) ...
... (de)hydrogenaion of the C—O or C—N bond proton released to the solution example: lactate dehydrogenase (lactate + NAD+ → → pyruvate + NADH +H+) ...
UBC Dairy Education and Research Centre
... Crude protein is made up of non-protein nitrogen (NPN) and true protein (TP) Non-protein nitrogen (NPN): nitrogen not in protein molecules (free peptides, free aa, nitrates, ammonia, etc) True protein (TP): nitrogen in the form of proteins (peptides linked together) ...
... Crude protein is made up of non-protein nitrogen (NPN) and true protein (TP) Non-protein nitrogen (NPN): nitrogen not in protein molecules (free peptides, free aa, nitrates, ammonia, etc) True protein (TP): nitrogen in the form of proteins (peptides linked together) ...
AA lecture 2 urea cycle
... H20 + fumarate + aa + NAD+ aspartate + -keto acid + NADH + H+ then aspartate + NH4+ + HCO3- + 3 ATP urea + fumarate + 2 H20 + 2 ADP + AMP + 4 Pi + H+ Four high energy phosphate bond equivalents are used for these reactions (- 4 ~P). Two NADH are produced. ...
... H20 + fumarate + aa + NAD+ aspartate + -keto acid + NADH + H+ then aspartate + NH4+ + HCO3- + 3 ATP urea + fumarate + 2 H20 + 2 ADP + AMP + 4 Pi + H+ Four high energy phosphate bond equivalents are used for these reactions (- 4 ~P). Two NADH are produced. ...
1. dia
... Chemistry of sulfur oxide formation • Sulfur trioxide at 482 oC transforms to sulfuric acid. Under the dew point sulfuric acid condensates on the structure materials (heat exchanger, stack wall ). The dew point of sulfuric acid depends on the SO3 and water content of the stack gas. Dew point of sul ...
... Chemistry of sulfur oxide formation • Sulfur trioxide at 482 oC transforms to sulfuric acid. Under the dew point sulfuric acid condensates on the structure materials (heat exchanger, stack wall ). The dew point of sulfuric acid depends on the SO3 and water content of the stack gas. Dew point of sul ...
video slide - Ionia Public Schools
... • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation ...
... • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation ...
01 - ALCA
... Remember that oxygen HAS TO BE AVAILBALE for Pyruvic acid to loose a carbon. After about 15-20 seconds of activity (depending on how ‘in shape’ the person is), oxygen is depleted and is no longer there to accept the carbon. What does Pyruvic acid do if no oxygen is around? It gives up! It becomes a ...
... Remember that oxygen HAS TO BE AVAILBALE for Pyruvic acid to loose a carbon. After about 15-20 seconds of activity (depending on how ‘in shape’ the person is), oxygen is depleted and is no longer there to accept the carbon. What does Pyruvic acid do if no oxygen is around? It gives up! It becomes a ...
Chapter 9
... Consumption of food & oxygen to produce CO2, water & energy C6H12O6 + 6O2 6CO2 + 6H2O + energy (ATP + heat) Exergonic rxn releases -686 kcal/mol using redox rxns ...
... Consumption of food & oxygen to produce CO2, water & energy C6H12O6 + 6O2 6CO2 + 6H2O + energy (ATP + heat) Exergonic rxn releases -686 kcal/mol using redox rxns ...
Cellular Respiration
... • Instead of this energy being released and wasted in a single explosive step, electrons cascade down the chain from one carrier molecule to the next in a series of redox reactions, losing a small amount of energy with each step until they finally reach oxygen, the terminal (final) electron acceptor ...
... • Instead of this energy being released and wasted in a single explosive step, electrons cascade down the chain from one carrier molecule to the next in a series of redox reactions, losing a small amount of energy with each step until they finally reach oxygen, the terminal (final) electron acceptor ...
Enter Topic Title in each section above
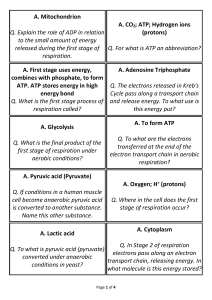
... to the small amount of energy released during the first stage of Q. For what is ATP an abbreviation? respiration. A. First stage uses energy, combines with phosphate, to form ATP. ATP stores energy in high energy bond Q. What is the first stage process of respiration called? ...
... to the small amount of energy released during the first stage of Q. For what is ATP an abbreviation? respiration. A. First stage uses energy, combines with phosphate, to form ATP. ATP stores energy in high energy bond Q. What is the first stage process of respiration called? ...
2 H + 1 / 2 O 2
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
video slide
... • NADH and FADH2 – Donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation – In the electron transport chain, electrons from NADH and FADH2 lose energy in several steps ...
... • NADH and FADH2 – Donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation – In the electron transport chain, electrons from NADH and FADH2 lose energy in several steps ...
Chapter 16 The Citric Acid Cycle
... • A 2-carbon unit Acetyl-CoA is added to the cycle • And two CO2 molecules leave (but they are different carbons…) • During the course of changes in the carbon skeleton and its oxidation state • And the transfer of energy to form GTP (aka. the “Canadian $”) and reducing power, as NADH and FADH2 • It ...
... • A 2-carbon unit Acetyl-CoA is added to the cycle • And two CO2 molecules leave (but they are different carbons…) • During the course of changes in the carbon skeleton and its oxidation state • And the transfer of energy to form GTP (aka. the “Canadian $”) and reducing power, as NADH and FADH2 • It ...
9-2 The Krebs Cycle and Electron Transport - holyoke
... What role does the Krebs cycle play in the cell? a. It breaks down glucose and releases its stored energy. b. It releases energy from molecules formed during glycolysis. c. It combines carbon dioxide and water into high-energy molecules. d. It breaks down ATP and NADH, releasing stored energy. Slide ...
... What role does the Krebs cycle play in the cell? a. It breaks down glucose and releases its stored energy. b. It releases energy from molecules formed during glycolysis. c. It combines carbon dioxide and water into high-energy molecules. d. It breaks down ATP and NADH, releasing stored energy. Slide ...
Archaea
... Hyperthermophilic Archaea and Bacteria are found on the deepest, shortest branches of the phylogenetic tree The oxidation of H2 is common to many hyperthermophiles and may have been the first energyyielding metabolism ...
... Hyperthermophilic Archaea and Bacteria are found on the deepest, shortest branches of the phylogenetic tree The oxidation of H2 is common to many hyperthermophiles and may have been the first energyyielding metabolism ...
Carbohydrate Metabolism
... accumulation of these protons in the space between the membranes creates a proton gradient with respect to the mitochondrial matrix. Also embedded in the inner mitochondrial membrane is an amazing protein pore complex called ...
... accumulation of these protons in the space between the membranes creates a proton gradient with respect to the mitochondrial matrix. Also embedded in the inner mitochondrial membrane is an amazing protein pore complex called ...
An Introduction to Metabolism and Energetics
... • The function of the citric acid cycle is: • To remove hydrogen atoms from organic molecules and transfer them to coenzymes • In the mitochondrion • Pyruvic acid reacts with NAD and coenzyme A (CoA) • Producing 1 CO2, 1 NADH, 1 acetyl-CoA ...
... • The function of the citric acid cycle is: • To remove hydrogen atoms from organic molecules and transfer them to coenzymes • In the mitochondrion • Pyruvic acid reacts with NAD and coenzyme A (CoA) • Producing 1 CO2, 1 NADH, 1 acetyl-CoA ...
Plankton
... • Net primary productivity is the amount of carbon dioxide removed via photosynthesis minus the amount of carbon dioxide released by respiration • Compensation depth refers to the depth in the water column at which the rate of photosynthesis equals the rate of respiration – Above this depth, phytopl ...
... • Net primary productivity is the amount of carbon dioxide removed via photosynthesis minus the amount of carbon dioxide released by respiration • Compensation depth refers to the depth in the water column at which the rate of photosynthesis equals the rate of respiration – Above this depth, phytopl ...
CHAPTER 4 | Solution Chemistry and the Hydrosphere
... e– + VO2+(aq) + 2 H+(aq) VO2+(aq) + H2O ( ) This reaction is a reduction. (d) As written, the reactant side has a charge of 0 and the product side has a charge of 10+. We need to add 10 electrons to the product side to balance the charge. I2(s) + 6 H2O ( ) 2 IO3–(aq) + 12 H+(aq) + 10 e– This rea ...
... e– + VO2+(aq) + 2 H+(aq) VO2+(aq) + H2O ( ) This reaction is a reduction. (d) As written, the reactant side has a charge of 0 and the product side has a charge of 10+. We need to add 10 electrons to the product side to balance the charge. I2(s) + 6 H2O ( ) 2 IO3–(aq) + 12 H+(aq) + 10 e– This rea ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)