Capability of Lactobacillus reuteri to Produce an Active Form of
... which share a structural architecture consisting of a corrin ring with a cobalt ion chelated at the core such as pseudovitamin B12 (Santos et al., 2007; Zempleni et al., 2007; Taga and Walker, 2008). This molecule differs from cobalamin in α-ligand, where it has adenine instead of 5,6-dimethylbenzim ...
... which share a structural architecture consisting of a corrin ring with a cobalt ion chelated at the core such as pseudovitamin B12 (Santos et al., 2007; Zempleni et al., 2007; Taga and Walker, 2008). This molecule differs from cobalamin in α-ligand, where it has adenine instead of 5,6-dimethylbenzim ...
04_Medicinal Natural..
... structure, often glycerol. Chemically, this is a triester of glycerol (Fig 5), an ester being the molecule formed from the reaction of an acid and an alcohol. Saturated and unsaturated fats differ in their energy content and melting point. Since an unsaturated fat contains fewer carbon-hydrogen bond ...
... structure, often glycerol. Chemically, this is a triester of glycerol (Fig 5), an ester being the molecule formed from the reaction of an acid and an alcohol. Saturated and unsaturated fats differ in their energy content and melting point. Since an unsaturated fat contains fewer carbon-hydrogen bond ...
Influence of temperature on the dynamics of ATP, ADP and non
... trees. The results of the nucleotides test indicated that endodormancy ended before the end of December (authors’ unpublished data), whereas changes in ATP concentration and ATP/ADP ratio did not occur until after the end of January (Figures 2 and 3). On the other hand, the dynamics of the changes s ...
... trees. The results of the nucleotides test indicated that endodormancy ended before the end of December (authors’ unpublished data), whereas changes in ATP concentration and ATP/ADP ratio did not occur until after the end of January (Figures 2 and 3). On the other hand, the dynamics of the changes s ...
Glutamate Dehydrogenases: Enzymology, Physiological
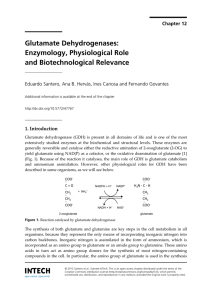
... Plants and microorganisms can utilise several inorganic nitrogen sources with different oxidation states such as N2 (by nitrogen-fixing bacteria and archaea), nitrate or nitrite, by reducing them to ammonium, which is subsequently assimilated. After formation of glutamate, the α-amino group can be t ...
... Plants and microorganisms can utilise several inorganic nitrogen sources with different oxidation states such as N2 (by nitrogen-fixing bacteria and archaea), nitrate or nitrite, by reducing them to ammonium, which is subsequently assimilated. After formation of glutamate, the α-amino group can be t ...
physiological reviews
... may be suggested that an anaerobic coupled phosphorylation at the electron transport level participates in the formation of high energy bonds in muscle. Such a reaction may account for the unexpectedly high yield of up to 1.9 molesof creatine phosphate per mole of lactic acid formed in living muscle ...
... may be suggested that an anaerobic coupled phosphorylation at the electron transport level participates in the formation of high energy bonds in muscle. Such a reaction may account for the unexpectedly high yield of up to 1.9 molesof creatine phosphate per mole of lactic acid formed in living muscle ...
Ketone body metabolism and cardiovascular disease - AJP
... in ketogenesis include lipolysis of fatty acids from triacylglycerols, transport to and across the hepatocyte plasma membrane, transport into mitochondria via allosterically regulated carnitine palmitoyltransferase 1, the -oxidation spiral, and the hormonal regulators of these processes, predominan ...
... in ketogenesis include lipolysis of fatty acids from triacylglycerols, transport to and across the hepatocyte plasma membrane, transport into mitochondria via allosterically regulated carnitine palmitoyltransferase 1, the -oxidation spiral, and the hormonal regulators of these processes, predominan ...
Enzyme Properties
... Sugars with at least four carbons can readily interconvert between the openchain forms we have drawn and fivemembered(furanose) or six-membered (pyranose) ring forms in which the carbonyl oxygen becomes part of the ring There are no C=O bonds in the ring forms ...
... Sugars with at least four carbons can readily interconvert between the openchain forms we have drawn and fivemembered(furanose) or six-membered (pyranose) ring forms in which the carbonyl oxygen becomes part of the ring There are no C=O bonds in the ring forms ...
IB Chemistry Online SAQ_Ans
... unstable in higher levels and rapidly emit radiation and fall back into lower energy levels. As the energy levels are fixed, the energy lost between any higher level and a lower level is also of a certain fixed value so the radiation emitted will only have certain fixed frequencies (i.e. specific co ...
... unstable in higher levels and rapidly emit radiation and fall back into lower energy levels. As the energy levels are fixed, the energy lost between any higher level and a lower level is also of a certain fixed value so the radiation emitted will only have certain fixed frequencies (i.e. specific co ...
Systematic metabolic analysis of recombinant Pichia pastoris UNIVERSITAT AUTÒNOMA DE BARCELONA
... energy and reducing power to synthesize all the cell components required. The metabolic pathways ensure the proper and efficient energy and reducing power supply to obtain the desired molecules. Therefore, a solid knowledge of the cellular metabolism may allow the efficiency enhance of a particular ...
... energy and reducing power to synthesize all the cell components required. The metabolic pathways ensure the proper and efficient energy and reducing power supply to obtain the desired molecules. Therefore, a solid knowledge of the cellular metabolism may allow the efficiency enhance of a particular ...
AN ABSTRACT OF THE THESIS OF (Name)
... growth of the above mentioned microorganisms impart the normal flavor and aroma to mixed-strain butter cultures. Much of the early research on the flavor of butter cultures was concerned with the organic acid production by mixed-strain starters. Lactic acid has long been known to be the major metabo ...
... growth of the above mentioned microorganisms impart the normal flavor and aroma to mixed-strain butter cultures. Much of the early research on the flavor of butter cultures was concerned with the organic acid production by mixed-strain starters. Lactic acid has long been known to be the major metabo ...
On the mechanism of action of the antifungal agent propionate
... on 100 mM propionate, we added 10 mM glucose to the medium to support initial growth. After total consumption of glucose cells were grown further for at least 12 h. Therefore, the wild-type strain was grown for 42 h, whereas the methylcitrate synthase deletion strain and the facB multi-copy strain w ...
... on 100 mM propionate, we added 10 mM glucose to the medium to support initial growth. After total consumption of glucose cells were grown further for at least 12 h. Therefore, the wild-type strain was grown for 42 h, whereas the methylcitrate synthase deletion strain and the facB multi-copy strain w ...
Molecular and General Genetics.
... system of the host plant. This eect is thought to result from the production of auxin-like compounds, such as indole-3-acetic acid (IAA), by the bacterium, because application of IAA mimics the eect of inoculation with the bacteria (for a review see Costacurta and Vanderleyden 1995). No mutant str ...
... system of the host plant. This eect is thought to result from the production of auxin-like compounds, such as indole-3-acetic acid (IAA), by the bacterium, because application of IAA mimics the eect of inoculation with the bacteria (for a review see Costacurta and Vanderleyden 1995). No mutant str ...
DISCOVERY OF ENZYMES RESPONSIBLE FOR AN ALTERNATE
... (2). Archaea are now positioned in a separate domain with bacteria and eukaryotes representing the other domains and creating the three domain biological classification system most prominent today. Their unique nature and independent evolutionary history prompts vigorous debate about not only the ev ...
... (2). Archaea are now positioned in a separate domain with bacteria and eukaryotes representing the other domains and creating the three domain biological classification system most prominent today. Their unique nature and independent evolutionary history prompts vigorous debate about not only the ev ...
ATP production in brain and liver mitochondria of Fischer
... three nucleotide binding sites that undergo a multiphase, catalytic cycle to produce ATP (43) from ADP and phosphate (i.e., oxidative phosphorylation). Because ATP is tightly bound to the F1 head in the mitochondrial membrane, a large amount of free energy is released during the rapid hydrolysis of ...
... three nucleotide binding sites that undergo a multiphase, catalytic cycle to produce ATP (43) from ADP and phosphate (i.e., oxidative phosphorylation). Because ATP is tightly bound to the F1 head in the mitochondrial membrane, a large amount of free energy is released during the rapid hydrolysis of ...
Presentation 2013-201307040352
... demand to sustain contractile oxidative phosphorylation (>95%) , function, basal metabolic with the remainder being derived processes, and ionic homeostasis. from glycolysis and GTP formation in • The heart has a relatively low ATP the tricarboxylic acid (TCA) cycle. content (5 μmol/g wet wt) and hi ...
... demand to sustain contractile oxidative phosphorylation (>95%) , function, basal metabolic with the remainder being derived processes, and ionic homeostasis. from glycolysis and GTP formation in • The heart has a relatively low ATP the tricarboxylic acid (TCA) cycle. content (5 μmol/g wet wt) and hi ...
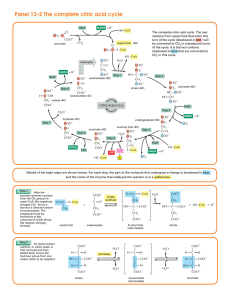
The Citric Acid Cycle
... referred to as oxidative phosphorylation, the high-transfer-potential electrons are transferred to oxygen to form water in a series of oxidation–reduction reactions. This transfer is highly exergonic, and the released energy is used to synthesize ATP. We will focus on the citric acid cycle in this s ...
... referred to as oxidative phosphorylation, the high-transfer-potential electrons are transferred to oxygen to form water in a series of oxidation–reduction reactions. This transfer is highly exergonic, and the released energy is used to synthesize ATP. We will focus on the citric acid cycle in this s ...
farmaceutski fakultet
... phase 1 enzymes that are expressed at high levels in the liver and localized to the ER. FMOs oxidize the nucleophilic nitrogen, sulfur, and phosphorus heteroatom of a variety of xenobiotics. FMOs are minor contributors to drug metabolism and generally produce benign metabolites. FMOs are not induced ...
... phase 1 enzymes that are expressed at high levels in the liver and localized to the ER. FMOs oxidize the nucleophilic nitrogen, sulfur, and phosphorus heteroatom of a variety of xenobiotics. FMOs are minor contributors to drug metabolism and generally produce benign metabolites. FMOs are not induced ...
Nicotine Increases Hepatic Oxygen Uptake in the Isolated Perfused
... A commonly reported effect of smoking is that upon commencement, individuals tend to lose weight, whereas cessation of smoking leads to weight gain (Troisi et al., 1991). This phenomenon also occurs in experimental animals (Ashakumary and Vijayammal, 1997) and indicates that nicotine affects energy ...
... A commonly reported effect of smoking is that upon commencement, individuals tend to lose weight, whereas cessation of smoking leads to weight gain (Troisi et al., 1991). This phenomenon also occurs in experimental animals (Ashakumary and Vijayammal, 1997) and indicates that nicotine affects energy ...
Analysis of the Role of Mitochondria of Sake in Fermentation Technologies
... (Takaya 2009). In Saccharomyces cerevisiae, cytochrome oxidase has been reported to reduce nitrite, which produces nitric oxide (NO) and mitochondrial electron potential (Castello et al. 2008), although the homolog of the nitrate reductase gene is not contained in the genome of S. cerevisiae. The ro ...
... (Takaya 2009). In Saccharomyces cerevisiae, cytochrome oxidase has been reported to reduce nitrite, which produces nitric oxide (NO) and mitochondrial electron potential (Castello et al. 2008), although the homolog of the nitrate reductase gene is not contained in the genome of S. cerevisiae. The ro ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)