14 - Lab Times
... the core of many cellular metabolic pathways. A year later, they linked amino acids into short peptides, the precursors of proteins. In 2000, George Cody from Washington’s Carnegie Institution reported creating pyruvate using a mineral catalyst under similar conditions – pyruvate with a three-carbon ...
... the core of many cellular metabolic pathways. A year later, they linked amino acids into short peptides, the precursors of proteins. In 2000, George Cody from Washington’s Carnegie Institution reported creating pyruvate using a mineral catalyst under similar conditions – pyruvate with a three-carbon ...
You Light Up My Life - Hillsborough Community College
... Lactate Fermentation • Carried out by certain bacteria • Electron transfer chain is in bacterial plasma membrane • Final electron acceptor is compound from environment (such as nitrate), not oxygen • ATP yield is low ...
... Lactate Fermentation • Carried out by certain bacteria • Electron transfer chain is in bacterial plasma membrane • Final electron acceptor is compound from environment (such as nitrate), not oxygen • ATP yield is low ...
Lecture 5
... The Free-Energy Change for a Reaction Determines Whether It Can Occur Although enzymes speed up reactions, they cannot by themselves force energetically unfavorable reactions to occur. According to the second law of thermodynamics, a chemical reaction can proceed only if it results in net increase ...
... The Free-Energy Change for a Reaction Determines Whether It Can Occur Although enzymes speed up reactions, they cannot by themselves force energetically unfavorable reactions to occur. According to the second law of thermodynamics, a chemical reaction can proceed only if it results in net increase ...
The Aerobic Fate of Pyruvate
... metabolism occurs. The 2 moles of NADH produced by glyceraldehyde-3-phosphate dehydrogenase are oxidized in the electron transport chain back to NAD+. The electron transport chain generates a proton gradient that drives the synthesis of 5 ATP molecules from ADP and Pi. Further more, the pyruvate for ...
... metabolism occurs. The 2 moles of NADH produced by glyceraldehyde-3-phosphate dehydrogenase are oxidized in the electron transport chain back to NAD+. The electron transport chain generates a proton gradient that drives the synthesis of 5 ATP molecules from ADP and Pi. Further more, the pyruvate for ...
Table of Contents - Milan Area Schools
... • NADH + H+ passes its hydrogen atoms to the NADH-Q reductase protein complex. • The NADH-Q reductase passes the hydrogens on to ubiquinone (Q), forming QH2. • The QH2 passes electrons to cytochrome c reductase complex which in turn passes them to ...
... • NADH + H+ passes its hydrogen atoms to the NADH-Q reductase protein complex. • The NADH-Q reductase passes the hydrogens on to ubiquinone (Q), forming QH2. • The QH2 passes electrons to cytochrome c reductase complex which in turn passes them to ...
chapter 9 cellular respiration: harvesting chemical
... The prosthetic group of each cytochrome is a heme group with an iron atom that accepts and donates electrons. The last cytochrome of the chain, cyt a3, passes its electrons to oxygen, which is very electronegative. Each oxygen atom also picks up a pair of hydrogen ions from the aqueous solution ...
... The prosthetic group of each cytochrome is a heme group with an iron atom that accepts and donates electrons. The last cytochrome of the chain, cyt a3, passes its electrons to oxygen, which is very electronegative. Each oxygen atom also picks up a pair of hydrogen ions from the aqueous solution ...
Chapter 9 – Cellular Respiration: Harvesting Chemical Energy
... Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
... Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
The Representative Elements: Group 5A Through 8A
... Biological nitrogen fixation requires a complex set of enzymes and a huge expenditure of ATP. Although the first stable product of the process is ammonia, this is quickly incorporated into protein and other organic nitrogen compounds. Industrial Fixation Under great pressure, at a temperature of 300 ...
... Biological nitrogen fixation requires a complex set of enzymes and a huge expenditure of ATP. Although the first stable product of the process is ammonia, this is quickly incorporated into protein and other organic nitrogen compounds. Industrial Fixation Under great pressure, at a temperature of 300 ...
The Smart Organism: Reinforcing NC Biology Curriculum for Ecology and Human Impacts
... starches. Sulfur is a component of proteins. Phosphorus and nitrogen help build DNA molecules. Without these substances, living things as we know them would not exist. These limiting nutrients cycle through the environment, between organisms, then back to the environment again by way of decomposers. ...
... starches. Sulfur is a component of proteins. Phosphorus and nitrogen help build DNA molecules. Without these substances, living things as we know them would not exist. These limiting nutrients cycle through the environment, between organisms, then back to the environment again by way of decomposers. ...
Biochemistry
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
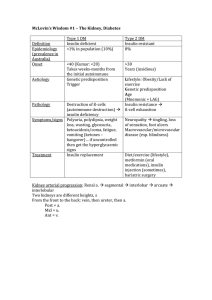
McLovin`s Wisdom #1 – The Kidney, Diabetes Type 1 DM Type 2
... At complex 4, 1/2O2 + 2H+ H2O (the H+s are reacted with oxygen to reduce it to water. Hence oxygen is needed). ATP synthase. 4H+ going through ATP synthase produce 1 ATP (3H+ go through there, and 1H+ used to transport the ATP back out into the intermembrane space – the outer mitochondrial membra ...
... At complex 4, 1/2O2 + 2H+ H2O (the H+s are reacted with oxygen to reduce it to water. Hence oxygen is needed). ATP synthase. 4H+ going through ATP synthase produce 1 ATP (3H+ go through there, and 1H+ used to transport the ATP back out into the intermembrane space – the outer mitochondrial membra ...
09_DetailLectOut_jkAR
... Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
... Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
Cellular Metabolism
... The entire process of synthesizing ATP in the electron transport system is called oxidative phosphorylation. The ATP is produced by chemiosmosis. 2. Chemiosmotic Theory of ATP Production – Chemi – chemical forces, Osmosis – pushing forces The chemiosmotic theory of ATP production is based on the fac ...
... The entire process of synthesizing ATP in the electron transport system is called oxidative phosphorylation. The ATP is produced by chemiosmosis. 2. Chemiosmotic Theory of ATP Production – Chemi – chemical forces, Osmosis – pushing forces The chemiosmotic theory of ATP production is based on the fac ...
Slides - Websupport1
... As soon as glucose is inside the cell, a phosphate is added to carbon number 6, and the new molecule is called glucose 6 phosphate. This reaction is called phosphorylation and it requires one ATP, enzyme ...
... As soon as glucose is inside the cell, a phosphate is added to carbon number 6, and the new molecule is called glucose 6 phosphate. This reaction is called phosphorylation and it requires one ATP, enzyme ...
Chapter 8 Enzymes: basic concepts and kinetics
... • In catabolism, some ATP is generated (substrate level phosphorylation), but most of the free energy is temporary stored in the reducing equivalents extracted from fuel molecules. • The reducing equivalents are transferred to NAD+ and FAD. NADH and FADH2 are formed. • Reducing equivalents are trans ...
... • In catabolism, some ATP is generated (substrate level phosphorylation), but most of the free energy is temporary stored in the reducing equivalents extracted from fuel molecules. • The reducing equivalents are transferred to NAD+ and FAD. NADH and FADH2 are formed. • Reducing equivalents are trans ...
Bio301 final exam 2005 with model answers
... Simultaneous nitrification and denitrification is a novel way or nitrogen removal from wastewater. Explain what the difficulties are to have both processes running in parallel and how SND can be accomplished. Nitrification involves the aerobic oxidation of ammonia, the key inorganic nitrogen compoun ...
... Simultaneous nitrification and denitrification is a novel way or nitrogen removal from wastewater. Explain what the difficulties are to have both processes running in parallel and how SND can be accomplished. Nitrification involves the aerobic oxidation of ammonia, the key inorganic nitrogen compoun ...
Oxygen Metabolism and Oxygen Toxicity
... Chemiosomitic Theory state that the free energy of electron transport is coupled to the pumping of protons from the matrix to the intermembrane space to create a pH gradient. This potential energy stored in this pH gradient is used to drive the synthesis of ATP. This process requires: 1. An intact i ...
... Chemiosomitic Theory state that the free energy of electron transport is coupled to the pumping of protons from the matrix to the intermembrane space to create a pH gradient. This potential energy stored in this pH gradient is used to drive the synthesis of ATP. This process requires: 1. An intact i ...
how cells release chemical energy
... __ 6. Select the process by which NADH and FADH, transfer electrons along a chain of acceptors to oxygen so as to form water and set up conditions for producing a large number of ATP molecules. [pp.1l4-llS] a. glycolysis b. the Krebs cycle c. acetyl-CoA formation d. fermentation pathways e. electron ...
... __ 6. Select the process by which NADH and FADH, transfer electrons along a chain of acceptors to oxygen so as to form water and set up conditions for producing a large number of ATP molecules. [pp.1l4-llS] a. glycolysis b. the Krebs cycle c. acetyl-CoA formation d. fermentation pathways e. electron ...
Cell Metabolism
... Cell Respiration: is the process of converting the chemical energy in food molecules (such as glucose) into a form the cell can use (ATP). ...
... Cell Respiration: is the process of converting the chemical energy in food molecules (such as glucose) into a form the cell can use (ATP). ...
video slide - Knappology
... Can produce ATP with or without oxygen, in aerobic or anaerobic conditions It is the ETC that requires oxygen (without it the e- are not pulled down the series of proteins and chemiosmosis fails) Glycolysis can couple with fermentation to produce ATP ...
... Can produce ATP with or without oxygen, in aerobic or anaerobic conditions It is the ETC that requires oxygen (without it the e- are not pulled down the series of proteins and chemiosmosis fails) Glycolysis can couple with fermentation to produce ATP ...
Chapter 20 – The Representative Elements
... Denitrification reduces nitrates to nitrogen gas, thus replenishing the atmosphere. Once again, bacteria are the agents. They live deep in soil and in aquatic sediments where conditions are anaerobic. They use nitrates as an alternative to oxygen for the final electron acceptor in their respiration. ...
... Denitrification reduces nitrates to nitrogen gas, thus replenishing the atmosphere. Once again, bacteria are the agents. They live deep in soil and in aquatic sediments where conditions are anaerobic. They use nitrates as an alternative to oxygen for the final electron acceptor in their respiration. ...
Lecture 4.
... atmosphere and the biosphere itself. It is these interactions which satisfy the needs of all living organisms such as food, shelter, water, and oxygen to respire, mates to reproduce etc, which are essential for sustained life on this planet. The complex system in which interactions between the diffe ...
... atmosphere and the biosphere itself. It is these interactions which satisfy the needs of all living organisms such as food, shelter, water, and oxygen to respire, mates to reproduce etc, which are essential for sustained life on this planet. The complex system in which interactions between the diffe ...
Document
... Fig. 9.11 overview of citric acid cycle (NADH, FADH2, ATP and CO2 produced) Fig. 9.12 closer look at the Citric acid cycle 9.4 Oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis Most of the ATP is produced in this Step of cell respiration! Oxygen is the final electro ...
... Fig. 9.11 overview of citric acid cycle (NADH, FADH2, ATP and CO2 produced) Fig. 9.12 closer look at the Citric acid cycle 9.4 Oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis Most of the ATP is produced in this Step of cell respiration! Oxygen is the final electro ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)