metabolism - anatomymodelimages
... 1. Vitamins – organic molecules; coenzyme or part; helps enzyme catalyze 2. Source – most we can’t make; bacteria K and pantothenic acid (B); vitamin D -a. Provitamin – like β carotene; used to make vitamin A (retinol) 3. Water soluble – C and B complex -a. Absorption – most with water in GI tract; ...
... 1. Vitamins – organic molecules; coenzyme or part; helps enzyme catalyze 2. Source – most we can’t make; bacteria K and pantothenic acid (B); vitamin D -a. Provitamin – like β carotene; used to make vitamin A (retinol) 3. Water soluble – C and B complex -a. Absorption – most with water in GI tract; ...
Summary of glycolysis (Embden
... hydrolysis of ATP. This irreversible step is the rate limiting reaction in glycolysis. The energy of bisphospho glycerate (1,3-BPG) is trapped to synthesize one ATP molecule with the help of bisphospho glycerate kinase. This is an example of substrate level phosphorylation (where energy is trapped ...
... hydrolysis of ATP. This irreversible step is the rate limiting reaction in glycolysis. The energy of bisphospho glycerate (1,3-BPG) is trapped to synthesize one ATP molecule with the help of bisphospho glycerate kinase. This is an example of substrate level phosphorylation (where energy is trapped ...
Glycogen Metabolism
... is the fate of most of NH3 channeled there. Urea → bloodstream → kidneys → urine ...
... is the fate of most of NH3 channeled there. Urea → bloodstream → kidneys → urine ...
PPT Oxidation
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
PPT Oxidation
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
Metabolism
... 9 acetyl coA’s through the citric acid cycle: 9 GTP, 67.5 ATP from 27 NADH and 13.5 ATP from 9 FADH2 Minus 2 ATP to start beta oxidation: 120 ATP Fat burns in a flame of carbohydrate Carbohydrate is needed Without sufficient oxaloacetate from carb to drive the citric acid cycle, the acetyl coA from ...
... 9 acetyl coA’s through the citric acid cycle: 9 GTP, 67.5 ATP from 27 NADH and 13.5 ATP from 9 FADH2 Minus 2 ATP to start beta oxidation: 120 ATP Fat burns in a flame of carbohydrate Carbohydrate is needed Without sufficient oxaloacetate from carb to drive the citric acid cycle, the acetyl coA from ...
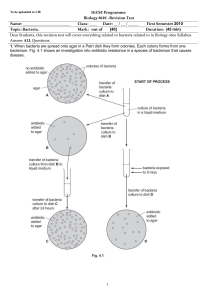
Bacteria - WordPress.com
... (b) Seven small paper discs were soaked in solutions of different antibiotics, A to G. The paper discs were placed on an agar plate which was evenly covered with growing bacteria. This was left for a short time. The results are shown in Fig. 3.4. ...
... (b) Seven small paper discs were soaked in solutions of different antibiotics, A to G. The paper discs were placed on an agar plate which was evenly covered with growing bacteria. This was left for a short time. The results are shown in Fig. 3.4. ...
CHAPTER 6
... • Organisms show a marked similarity in their major metabolic pathways • All life descended from a common ancestral form – For example, Glycolysis, the metabolic pathway by which energy is released from glucose and captured in the form of ATP under anaerobic condition, is common to almost every cell ...
... • Organisms show a marked similarity in their major metabolic pathways • All life descended from a common ancestral form – For example, Glycolysis, the metabolic pathway by which energy is released from glucose and captured in the form of ATP under anaerobic condition, is common to almost every cell ...
Fatty Acid Catabolism
... 1. Which lipid form is transported across the inner mitochondrial membrane before β‐oxidation? A) Acylcarnitine. B) Fatty acyl CoA. C) Acetoacetyl CoA. D) Lysophospholipid CoA. 2. There are four steps in the β‐oxidation pathway. Some reaction types are listed below. Give the proper reaction t ...
... 1. Which lipid form is transported across the inner mitochondrial membrane before β‐oxidation? A) Acylcarnitine. B) Fatty acyl CoA. C) Acetoacetyl CoA. D) Lysophospholipid CoA. 2. There are four steps in the β‐oxidation pathway. Some reaction types are listed below. Give the proper reaction t ...
Chapter 8 – an introduction to metabolism
... 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized to acetyl CoA, what molecules are produced, and how this process links glycolysis to the citric acid cycle. 12. List the products of the citric acid cycle. Explai ...
... 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized to acetyl CoA, what molecules are produced, and how this process links glycolysis to the citric acid cycle. 12. List the products of the citric acid cycle. Explai ...
Chapter 8 - Energy and Enzymes
... ions into the thylakoid. The energy of their movement back into the stroma by osmotic pressure is used to produce ATP. The enzyme that uses a hydrogen ion concentration gradient to phosphorylate ADP is ATP synthase. Photophosphorylation and oxidative phosphorylation (discussed above) both use an osm ...
... ions into the thylakoid. The energy of their movement back into the stroma by osmotic pressure is used to produce ATP. The enzyme that uses a hydrogen ion concentration gradient to phosphorylate ADP is ATP synthase. Photophosphorylation and oxidative phosphorylation (discussed above) both use an osm ...
Bio AP chp 9 notes
... Under anaerobic conditions, various fermentation pathways generate ATP by glycolysis and recycle NAD+ by transferring electrons from NADH to pyruvate or derivatives of pyruvate. ...
... Under anaerobic conditions, various fermentation pathways generate ATP by glycolysis and recycle NAD+ by transferring electrons from NADH to pyruvate or derivatives of pyruvate. ...
Tricarboxylic acid cycle
... 2. Isocitrate dehydrogenase: Inhibited by ATP and NADH and activated by ADP 3. -KG dehydrogenase inhibited by NADH & succinyl CoA The availability of ADP: Important for proceeding the TCA cycle if not oxidation of NADH and FADH2 through election transport chain stops. Accumulation of NADH and FADH2 ...
... 2. Isocitrate dehydrogenase: Inhibited by ATP and NADH and activated by ADP 3. -KG dehydrogenase inhibited by NADH & succinyl CoA The availability of ADP: Important for proceeding the TCA cycle if not oxidation of NADH and FADH2 through election transport chain stops. Accumulation of NADH and FADH2 ...
16-18 Cellular respiration
... Fermentation – an ATP-producing catabolic pathway in which both electron donors and acceptors are organic compounds. Can be an anaerobic process Results in partial degradation of sugars Cellular respiration – an ATP-producing catabolic process in which the ultimate electron acceptor is an inorganic ...
... Fermentation – an ATP-producing catabolic pathway in which both electron donors and acceptors are organic compounds. Can be an anaerobic process Results in partial degradation of sugars Cellular respiration – an ATP-producing catabolic process in which the ultimate electron acceptor is an inorganic ...
the krebs cycle
... Stage 1: ATP is being broken down into ADP + Pi. The bond between the terminal inorganic phosphate and the second is broken. This releases energy. Stage 2: The energy released from ATP is transferred into another cellular process. In this example it is the contract of muscle fibres. Stage 3: If ther ...
... Stage 1: ATP is being broken down into ADP + Pi. The bond between the terminal inorganic phosphate and the second is broken. This releases energy. Stage 2: The energy released from ATP is transferred into another cellular process. In this example it is the contract of muscle fibres. Stage 3: If ther ...
Lecture Slides
... • The molecules of the electron transport chain are built into the inner membranes of mitochondria. – The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the inner ...
... • The molecules of the electron transport chain are built into the inner membranes of mitochondria. – The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the inner ...
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL
... ° Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
... ° Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL
... Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
... Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. ...
Armageddon Final Project
... Well competition is the struggle of 2 organisms for something. Well we too need this in our biome and we have that I will show you one example or multiple so you know we have competition going on right here. 1st example of competition is when the Cheetah competes vs.. the Hyena for the Impala an ...
... Well competition is the struggle of 2 organisms for something. Well we too need this in our biome and we have that I will show you one example or multiple so you know we have competition going on right here. 1st example of competition is when the Cheetah competes vs.. the Hyena for the Impala an ...
Degree of reduction
... Messenger RNA molecules carry messages from DNA to other parts of the cell. These messages are read in the ribosome with the help of ribosomal RNA. Finally transfer RNA assists in the translation of the genetic code at the ribosome. ...
... Messenger RNA molecules carry messages from DNA to other parts of the cell. These messages are read in the ribosome with the help of ribosomal RNA. Finally transfer RNA assists in the translation of the genetic code at the ribosome. ...
Volatile Fatty Acids
... Major VFA: acetic acid; propionic acid; butyric acid. Major VFAs are absorbed and used as primary energy source by ruminants. The tissue use of VFA is lower than tissue use of the sugars (e.g., glucose). ~10 % of energy consumed goes towards fermentation (methane). ...
... Major VFA: acetic acid; propionic acid; butyric acid. Major VFAs are absorbed and used as primary energy source by ruminants. The tissue use of VFA is lower than tissue use of the sugars (e.g., glucose). ~10 % of energy consumed goes towards fermentation (methane). ...
Biological Pathways II: Metabolic Pathways
... and from light is transformed into ATP - the energy currency A large amount of free energy is liberated when ATP is hydrolyzed to ADP & Pi, or ATP to AMP & PPi ATP + H2O ADP + Pi ...
... and from light is transformed into ATP - the energy currency A large amount of free energy is liberated when ATP is hydrolyzed to ADP & Pi, or ATP to AMP & PPi ATP + H2O ADP + Pi ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)