Lecture 4 - microeco
... Molecular records of the biosphere: databases containing genome and protein sequences. Evolutionary models based on functional bioinformatics. How large is the essential genome ? ...
... Molecular records of the biosphere: databases containing genome and protein sequences. Evolutionary models based on functional bioinformatics. How large is the essential genome ? ...
13.1 Ecologists Study Relationships
... • Producers get their energy from non-living resources. • Producers are also called autotrophs because they make their own food. ...
... • Producers get their energy from non-living resources. • Producers are also called autotrophs because they make their own food. ...
Lecture 3: Glycolysis Part 2 - University of California, Berkeley
... reaction. The oxidation is on the carbon. This is aided by the abstraction of the proton on the -OH group, ending up with a thioester. Thioesters. The hydrolysis of thioesters is much more strongly downhill than the hydrolysis of simple esters. Oxygen-based esters like this give resonance stabilizat ...
... reaction. The oxidation is on the carbon. This is aided by the abstraction of the proton on the -OH group, ending up with a thioester. Thioesters. The hydrolysis of thioesters is much more strongly downhill than the hydrolysis of simple esters. Oxygen-based esters like this give resonance stabilizat ...
Ecosystems - Science EOG
... In some cases, the species structure of an ecosystem is changed rapidly by a disturbance, such as a forest fire. If the ecosystem becomes unstable, primary or secondary succession can result. If succession begins in a new, unoccupied habitat where there is no soil present, it is called primary succ ...
... In some cases, the species structure of an ecosystem is changed rapidly by a disturbance, such as a forest fire. If the ecosystem becomes unstable, primary or secondary succession can result. If succession begins in a new, unoccupied habitat where there is no soil present, it is called primary succ ...
Benthic Ecology and Demersal Resources
... to replenish the population is important from fishery perspective How these are distributed over different type of sea bed and particular season favors any particular group of organisms and in turn these support specific fishery is an important issue ...
... to replenish the population is important from fishery perspective How these are distributed over different type of sea bed and particular season favors any particular group of organisms and in turn these support specific fishery is an important issue ...
HONORS BIOLOGY MIDTERM EXAM STUDY GUIDE 2016
... b) Explain the role of hydrogen ions (H+) in the electron transport chain. c) Explain the role of ATP synthase in the electron transport chain. 42. Identify the ATP yield for each step of aerobic cellular respiration and the approximate total ATP yield from one molecule of glucose. 43. Explain the r ...
... b) Explain the role of hydrogen ions (H+) in the electron transport chain. c) Explain the role of ATP synthase in the electron transport chain. 42. Identify the ATP yield for each step of aerobic cellular respiration and the approximate total ATP yield from one molecule of glucose. 43. Explain the r ...
Ch 26 Powerpoint
... • Energy from step-wise release powers pumping H+ into intermembrane space by chemiosmosis – The concentration of H+ outside > than that inside – this produces an electrostatic gradient and a net voltage. – Since it is positive charges – it is called proton motive force instead of electromotive forc ...
... • Energy from step-wise release powers pumping H+ into intermembrane space by chemiosmosis – The concentration of H+ outside > than that inside – this produces an electrostatic gradient and a net voltage. – Since it is positive charges – it is called proton motive force instead of electromotive forc ...
2 ATP - HCC Learning Web
... • Some ATP is also formed directly during glycolysis and the citric acid cycle by substrate-level phosphorylation, in which an enzyme transfers a phosphate group from an organic substrate molecule to ADP, forming only 4 ATP. ...
... • Some ATP is also formed directly during glycolysis and the citric acid cycle by substrate-level phosphorylation, in which an enzyme transfers a phosphate group from an organic substrate molecule to ADP, forming only 4 ATP. ...
Biology_1_&_2_files/2 Biochemistry ACADEMIC
... a series of reactions using many enzymes to capture energy in the form of ATP molecules. The enzymes reduce the activation energy so much ...
... a series of reactions using many enzymes to capture energy in the form of ATP molecules. The enzymes reduce the activation energy so much ...
Carbohydrate Metabolism - BITS Academic Resource Center
... How do we use food components in catabolic and anabolic pathways? Involves specific chemical reactions: - Each reaction is catalyzed by a specific enzyme. - Other compounds, besides those being directly metabolized, are required as intermediates or catalysts in metabolic reactions - adenosine triph ...
... How do we use food components in catabolic and anabolic pathways? Involves specific chemical reactions: - Each reaction is catalyzed by a specific enzyme. - Other compounds, besides those being directly metabolized, are required as intermediates or catalysts in metabolic reactions - adenosine triph ...
Biotech Lect-10 - ASAB-NUST
... Microbial Biotechnology in Food and Agriculture • reduction in the reliance on chemical treatments to control weeds by engineering herbicide tolerance into crops • production of products that have high yield and enhanced nutritional value ...
... Microbial Biotechnology in Food and Agriculture • reduction in the reliance on chemical treatments to control weeds by engineering herbicide tolerance into crops • production of products that have high yield and enhanced nutritional value ...
Chapter 1: Prelude
... molecule storing and transporting engergy, ATP. Further processes based on biochemical reactions are folding of proteins, recognition of substrates by enzymes, and detection of signal molecules. These and many more functions of biochemical molecules can be explained by their threedimensional structu ...
... molecule storing and transporting engergy, ATP. Further processes based on biochemical reactions are folding of proteins, recognition of substrates by enzymes, and detection of signal molecules. These and many more functions of biochemical molecules can be explained by their threedimensional structu ...
OXIDATIVE PHOSPHORYLATION
... The b were engineered to contain N-terminal polyhistidine tags, The tags allowed the a3b3 assembly to be immobilized on a glass surface that had been coated with nickel ions. The g subunit was linked to a fluorescently labeled actin filament The addition of ATP caused the g to rotate in a counterclo ...
... The b were engineered to contain N-terminal polyhistidine tags, The tags allowed the a3b3 assembly to be immobilized on a glass surface that had been coated with nickel ions. The g subunit was linked to a fluorescently labeled actin filament The addition of ATP caused the g to rotate in a counterclo ...
METABOLISM: BASIC CONSEPTS & DESIGN
... 7. Protein turnover and amino acids metabolism (ch. 23) 8. Synthesizing the molecules of life: 9. Biosynthesis of Amino acids (Ch.24) ...
... 7. Protein turnover and amino acids metabolism (ch. 23) 8. Synthesizing the molecules of life: 9. Biosynthesis of Amino acids (Ch.24) ...
Balancing Reaction Equations Oxidation State Reduction
... spectators. Reduce coefficients to lowest terms. Be sure the equation is balanced for both atoms and charge. ...
... spectators. Reduce coefficients to lowest terms. Be sure the equation is balanced for both atoms and charge. ...
How did LUCA make a living?
... of CO2 to acetyl CoA without the participation of ATP or any other triphosphate. Transition metal sulphides abound in the methanogen version of the acetyl CoA pathway,(6) but the universal energy currency ATP is missing. Instead, thioesters like acetyl CoA are central to the bioenergetics of the mos ...
... of CO2 to acetyl CoA without the participation of ATP or any other triphosphate. Transition metal sulphides abound in the methanogen version of the acetyl CoA pathway,(6) but the universal energy currency ATP is missing. Instead, thioesters like acetyl CoA are central to the bioenergetics of the mos ...
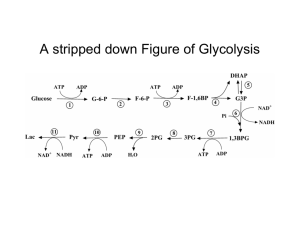
Figure 17-3 Degradation of glucose via the glycolytic pathway.
... by NADH. Thus, no net oxidation occurs in glycolysis = fermentation; another organic serving as electron acceptor. •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skele ...
... by NADH. Thus, no net oxidation occurs in glycolysis = fermentation; another organic serving as electron acceptor. •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skele ...
Are You suprised ?
... For questions 21-26, fill in the blanks 21. In plant and animal cells, ________________________________ is the site where the most of the cells ATP is generated. 22. _____________________________________ is widely claimed to be the most abundant protein on earth. 23. For every three molecules of CO2 ...
... For questions 21-26, fill in the blanks 21. In plant and animal cells, ________________________________ is the site where the most of the cells ATP is generated. 22. _____________________________________ is widely claimed to be the most abundant protein on earth. 23. For every three molecules of CO2 ...
MS Chapter 3 Powerpoint
... • Some recycle nutrients back to producers by decomposing the wastes and remains of organisms ...
... • Some recycle nutrients back to producers by decomposing the wastes and remains of organisms ...
determining evolutionary relationships using
... In this course you have learned that living organisms regardless of species are united by one common element…….we all have hereditary material in our cells which holds the instructions for making protein. The cells of all organisms recognize the language of DNA and thus are capable of producing prot ...
... In this course you have learned that living organisms regardless of species are united by one common element…….we all have hereditary material in our cells which holds the instructions for making protein. The cells of all organisms recognize the language of DNA and thus are capable of producing prot ...
Energy metabolism
... of fat in liver leading to hyperlipidemia or fatty liver. Since CAC is inhibited, availability of oxalloacetate is also limited, this leads to the inhibition of gluconeogenesis leading to hypoglycemia. Excess of NADH accelerates lactate dh to produce lactic acid leading to ...
... of fat in liver leading to hyperlipidemia or fatty liver. Since CAC is inhibited, availability of oxalloacetate is also limited, this leads to the inhibition of gluconeogenesis leading to hypoglycemia. Excess of NADH accelerates lactate dh to produce lactic acid leading to ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)