Introduction in energy systems - Faculty of Mechanical Engineering
... surrounding objects (Kelvin statement). Third law of thermodynamics The system is not possible cooled, in a finite number of steps, to absolute zero. ...
... surrounding objects (Kelvin statement). Third law of thermodynamics The system is not possible cooled, in a finite number of steps, to absolute zero. ...
Bagian 2 termodinamika
... Gulf of Mexico: Another free loader idea is to generate power as follows. Water on top of the Gulf of Mexico is quite warm but deep down the water is cold. The plan is to heat some gas with warm water from the top so it will expand, and then cool the gas with water from the bottom so it will contrac ...
... Gulf of Mexico: Another free loader idea is to generate power as follows. Water on top of the Gulf of Mexico is quite warm but deep down the water is cold. The plan is to heat some gas with warm water from the top so it will expand, and then cool the gas with water from the bottom so it will contrac ...
New Microsoft Office Word Document
... Surrounding:- Part of universe apart from system Universe:- System along with all the surroundings Boundary:- Walls that separate System from Surroundings Equilibrium:- A state of dynamics wherein all observable properties are constant Thermodynamic Equilibrium:- A system in which all macroscopic pr ...
... Surrounding:- Part of universe apart from system Universe:- System along with all the surroundings Boundary:- Walls that separate System from Surroundings Equilibrium:- A state of dynamics wherein all observable properties are constant Thermodynamic Equilibrium:- A system in which all macroscopic pr ...
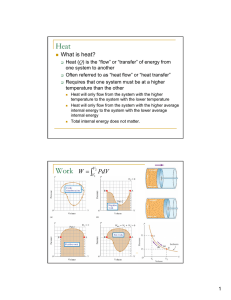
Heat Work
... A P-V diagram for a reversible heat engine in which 1.00 mole of argon, a nearly ideal monatomic gas, is initially at STP (point a). Points b and c are on an isothermal. If the engine produces positive work, a) is the cycle clockwise or counter clockwise, b) what is the efficiency of the cycle? ...
... A P-V diagram for a reversible heat engine in which 1.00 mole of argon, a nearly ideal monatomic gas, is initially at STP (point a). Points b and c are on an isothermal. If the engine produces positive work, a) is the cycle clockwise or counter clockwise, b) what is the efficiency of the cycle? ...
Initial state Equilibrium state
... the number of water molecules NWB and the number of total lattice sites MB. (c) Use a lattice model to express the entropy of system A at the start of the experiment in terms of the number of water molecules in system A, NwA, the number of solute particles, Ns, and the total number of lattice sites ...
... the number of water molecules NWB and the number of total lattice sites MB. (c) Use a lattice model to express the entropy of system A at the start of the experiment in terms of the number of water molecules in system A, NwA, the number of solute particles, Ns, and the total number of lattice sites ...
Defects - Script
... Purely mechanical systems (consisting of non-interacting mass points) would be in equilibrium for the lowest possible internal energy, i.e. for a minimum in their potential energy and no movement - just lying still at the lowest possible point. But thermodynamic systems consisting of many interactin ...
... Purely mechanical systems (consisting of non-interacting mass points) would be in equilibrium for the lowest possible internal energy, i.e. for a minimum in their potential energy and no movement - just lying still at the lowest possible point. But thermodynamic systems consisting of many interactin ...
system
... Heat and work are forms of energy that are transformed into other forms of energy. If friction is eliminated, work is efficiently transformed to potential, kinetic, electrical and heat energy by a conversion ratio of upto 100% Heat on the other hand is readily lost to the surroundings and its conver ...
... Heat and work are forms of energy that are transformed into other forms of energy. If friction is eliminated, work is efficiently transformed to potential, kinetic, electrical and heat energy by a conversion ratio of upto 100% Heat on the other hand is readily lost to the surroundings and its conver ...
Thermochemistry
... Second Law of Thermodynamics One statement defining the second law is that a spontaneous natural processes tend to even out the energy gradients in a isolated system. Can be quantified based on the entropy of the system, S, such that S is at a maximum when energy is most uniform. Can also be vi ...
... Second Law of Thermodynamics One statement defining the second law is that a spontaneous natural processes tend to even out the energy gradients in a isolated system. Can be quantified based on the entropy of the system, S, such that S is at a maximum when energy is most uniform. Can also be vi ...
Chemical Thermodynamics
... • If energy can be dispersed over a larger number of particles, it will be. • The greater the number of molecules and the higher the total energy of the system, the less likely the energy will be concentrated in a few molecules. Therefore, the energy will be more dispersed. • So, the final state of ...
... • If energy can be dispersed over a larger number of particles, it will be. • The greater the number of molecules and the higher the total energy of the system, the less likely the energy will be concentrated in a few molecules. Therefore, the energy will be more dispersed. • So, the final state of ...
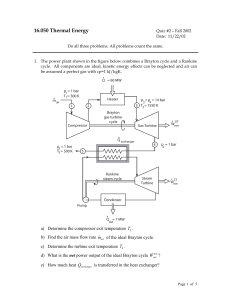
16.050 Thermal Energy
... g) What is the overall thermal efficiency of the combined cycle? 2. The sketch below shows a perfectly insulated container with two compartments separated by a non-adiabatic, frictionless piston. Both compartments are at the same pressure (pA = pB) and contain an equal amount of the same gas (mA = m ...
... g) What is the overall thermal efficiency of the combined cycle? 2. The sketch below shows a perfectly insulated container with two compartments separated by a non-adiabatic, frictionless piston. Both compartments are at the same pressure (pA = pB) and contain an equal amount of the same gas (mA = m ...
Chemical Thermodynamic
... function, the difference in internal energy from A to B or B to A should be same. If not, suppose the internal energy increases by an amount E while going from A to B. Now suppose the internal energy decreases by an amount E' while returning from B to A. Let E > E', that means after returning t ...
... function, the difference in internal energy from A to B or B to A should be same. If not, suppose the internal energy increases by an amount E while going from A to B. Now suppose the internal energy decreases by an amount E' while returning from B to A. Let E > E', that means after returning t ...
The Thermodynamic Potentials
... systems, there will be a redistribution of energy between the two systems. Again, if holes are punched in the piston, there will be a redistribution of matter (and also of energy) between the two systems. ...
... systems, there will be a redistribution of energy between the two systems. Again, if holes are punched in the piston, there will be a redistribution of matter (and also of energy) between the two systems. ...
Nonextensivity-Nonintensivity
... In order to explain the nature of nonextensivity of nanoscale systems the following discussion is presented: In thermodynamics, properties (variables) are classified as being either extensive or intensive. When properties of a system are independent of the number of particles present in the system, ...
... In order to explain the nature of nonextensivity of nanoscale systems the following discussion is presented: In thermodynamics, properties (variables) are classified as being either extensive or intensive. When properties of a system are independent of the number of particles present in the system, ...
THERMODYNAMICS OF NONCOMMUTATIVE BLACK HOLE
... Noncommutativity is expected to be relevant at the Planck scale where it is known that usual semi classical Considerations break down. It is therefore reasonable to expect that noncommutativity would modify the entropy. If we consider the quantization of gravity then we have to consider the notion o ...
... Noncommutativity is expected to be relevant at the Planck scale where it is known that usual semi classical Considerations break down. It is therefore reasonable to expect that noncommutativity would modify the entropy. If we consider the quantization of gravity then we have to consider the notion o ...
Kinetic Theory
... We will often write the free energy , G, in terms of some changeable parameter, x, of the open system, for example a protein’s size, or the number of bound factors to DNA etc. Equilibrium occurs when the free energy is at a minimum with respect to this parameter, ...
... We will often write the free energy , G, in terms of some changeable parameter, x, of the open system, for example a protein’s size, or the number of bound factors to DNA etc. Equilibrium occurs when the free energy is at a minimum with respect to this parameter, ...
Chapter 17 - Richsingiser.com
... 192.3 J/mol.K assuming that DHo and DSo do not change with temperature, calculate DGo at 1000 ...
... 192.3 J/mol.K assuming that DHo and DSo do not change with temperature, calculate DGo at 1000 ...
C -- needs 4 e`s to complete its outer shell --
... states (25°C, 1 atmosphere pressure, in their most stable form) is 0. The free energy of formation of a compound, DGf, is the change in G for formation of one mole from its component elements, all in their standard states. Then for a chemical reaction DG° = DGf (products) - DGf (reactants). Biochemi ...
... states (25°C, 1 atmosphere pressure, in their most stable form) is 0. The free energy of formation of a compound, DGf, is the change in G for formation of one mole from its component elements, all in their standard states. Then for a chemical reaction DG° = DGf (products) - DGf (reactants). Biochemi ...
Introduction to Heat Transfer
... derivations. Key terms, such as enthalpy and free energies, as well as experimental variables, such as the coefficient of thermal expansion and compressibility, will be defined. Maxwell relations will then be used to incorporate the experimental variables into expressions for thermodynamic parameter ...
... derivations. Key terms, such as enthalpy and free energies, as well as experimental variables, such as the coefficient of thermal expansion and compressibility, will be defined. Maxwell relations will then be used to incorporate the experimental variables into expressions for thermodynamic parameter ...
• Thermodynamics, what is it? • System, Surrounding and Boundary
... A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. For example, if liquid water and water vapor form a system with two phases, the system can be regarded as a pure substance because each phase has the same composition. The nature of phases ...
... A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. For example, if liquid water and water vapor form a system with two phases, the system can be regarded as a pure substance because each phase has the same composition. The nature of phases ...
Introduction to Thermodynamics
... L1: dU = dQ - PdV L2: dQ ≤ TdS dG = dU + PdV + vdP - TdS - SdT Substitute L1, take constant P,T dG = dQ - TdS This is always less than zero by L2. ...
... L1: dU = dQ - PdV L2: dQ ≤ TdS dG = dU + PdV + vdP - TdS - SdT Substitute L1, take constant P,T dG = dQ - TdS This is always less than zero by L2. ...
Biogeochemical cycles and thermodynamics
... Living systems are at non-equilibrium and the bonds between C, H, N, O, P are reduced or electron rich. Living systems must continuously process energy to counteract the basic laws of thermodynamics. Photoautotrophs do this by capturing light energy. Heterotrophs obtain metabolic energy by capitaliz ...
... Living systems are at non-equilibrium and the bonds between C, H, N, O, P are reduced or electron rich. Living systems must continuously process energy to counteract the basic laws of thermodynamics. Photoautotrophs do this by capturing light energy. Heterotrophs obtain metabolic energy by capitaliz ...
Second Law of thermodynamics
... • It is one of the great laws of physics • Its validity rests on experiments (such as Joule’s) in which no exceptions have been seen • Internal energy is the sum total of all the energy the molecules of the system. It is a property of a system like pressure, volume and temperature • Work and heat ar ...
... • It is one of the great laws of physics • Its validity rests on experiments (such as Joule’s) in which no exceptions have been seen • Internal energy is the sum total of all the energy the molecules of the system. It is a property of a system like pressure, volume and temperature • Work and heat ar ...
1. Discuss the following concepts
... 1. Discuss the following concepts (just writing formulas is not enough, use words) Enthropic principle Closed system Subsystem Distribution function Microcanonical distribution function 2. Consider N identical non-interacting 1D harmonic oscillators. The energy levels of the system will be given by: ...
... 1. Discuss the following concepts (just writing formulas is not enough, use words) Enthropic principle Closed system Subsystem Distribution function Microcanonical distribution function 2. Consider N identical non-interacting 1D harmonic oscillators. The energy levels of the system will be given by: ...
Chapter Summary
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...