Chapter 6 Thermodynamics and the Equations of Motion
... meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed to discuss the waves in your b ...
... meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed to discuss the waves in your b ...
Slide 1
... Debye theory, Cv is proportional to T3 and so the heat capacity of a metal takes the form Cv = AT + BT3, where the first term is the electronic contribution and the second is associated with the crystal lattice. • At sufficiently low temperatures, the AT term can dominate, as the sketch of Figure 19 ...
... Debye theory, Cv is proportional to T3 and so the heat capacity of a metal takes the form Cv = AT + BT3, where the first term is the electronic contribution and the second is associated with the crystal lattice. • At sufficiently low temperatures, the AT term can dominate, as the sketch of Figure 19 ...
Chapter 6
... waves in the atmosphere or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed ...
... waves in the atmosphere or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed ...
T - Massey University
... The two forms of energy that influence this internal energy are heat, either transferred to the system from a source at higher temperature or lost to a sink at lower temperature and work, which can increase the internal energy if work is done on the system by its surroundings, or decrease the intern ...
... The two forms of energy that influence this internal energy are heat, either transferred to the system from a source at higher temperature or lost to a sink at lower temperature and work, which can increase the internal energy if work is done on the system by its surroundings, or decrease the intern ...
1 Thermodynamics All biochemical and cellular processes obey the
... Many chemical reactions occur spontaneously. The spontaneity of a reaction cannot be predicted based simply on entropic considerations. (For example, the reaction 2 H2 + O2 → 2 H2O is usually spontaneous, in spite of the lower entropy associated with fewer products than reactants. Note, however, tha ...
... Many chemical reactions occur spontaneously. The spontaneity of a reaction cannot be predicted based simply on entropic considerations. (For example, the reaction 2 H2 + O2 → 2 H2O is usually spontaneous, in spite of the lower entropy associated with fewer products than reactants. Note, however, tha ...
PY2P10 Finn Problems Chap 4
... internalenergyof the sand?(c)What is the entropychangeassociated with this LU at constant7? The sanddoesno externalwork as it denot its volume.) formswhenit hits the pavement:only its shapechanges, 5.8 One mole of an ideal gas undergoesa free expansiontripling its volume.What is theentropychangeof ( ...
... internalenergyof the sand?(c)What is the entropychangeassociated with this LU at constant7? The sanddoesno externalwork as it denot its volume.) formswhenit hits the pavement:only its shapechanges, 5.8 One mole of an ideal gas undergoesa free expansiontripling its volume.What is theentropychangeof ( ...
Thermodynamics
... Positive when system gains heat Negative when system loses heat W = net work done by ...
... Positive when system gains heat Negative when system loses heat W = net work done by ...
Elastomers and aging
... after aging the procession of aging can be monitored in "real time." This testing can be done using the "multi-wave" testing procedure developed by Rheometrics (ref. 3). Logically, the new Monsanto - RPA2000 instrument can also be programmed to develop the same data, as both instruments are operatin ...
... after aging the procession of aging can be monitored in "real time." This testing can be done using the "multi-wave" testing procedure developed by Rheometrics (ref. 3). Logically, the new Monsanto - RPA2000 instrument can also be programmed to develop the same data, as both instruments are operatin ...
Thermodynamics - StrikerPhysics
... • Thermo (heat) dynamics (transfer) • Thermodynamic systems describe many many particles (molecules) which obey Newton’s laws for dynamics but which would be difficult to analyze due to their numbers. • We use macroscopic means for analysis of these systems of many particles - involving quantities s ...
... • Thermo (heat) dynamics (transfer) • Thermodynamic systems describe many many particles (molecules) which obey Newton’s laws for dynamics but which would be difficult to analyze due to their numbers. • We use macroscopic means for analysis of these systems of many particles - involving quantities s ...
Some useful Statistical Thermodynamics 1 Introduction
... The second law states that the number of accessible micro-states of an isolated system, Ω, never decreases. If we consider subsystem A to contain and ideal atomic gas, then the number of accessible micro-states of A is simply the number of places in space that may be occupied by the gas atoms. Thus, ...
... The second law states that the number of accessible micro-states of an isolated system, Ω, never decreases. If we consider subsystem A to contain and ideal atomic gas, then the number of accessible micro-states of A is simply the number of places in space that may be occupied by the gas atoms. Thus, ...
Thermodynamics
... of entropy is that it appears to depend on a path variable. But the fact that the heat transfer isn't just any type of heat transfer, but only along a special kind of path, allows it to be a state variable. Put another way, calculating changes in entropy from one state to another, we must construct ...
... of entropy is that it appears to depend on a path variable. But the fact that the heat transfer isn't just any type of heat transfer, but only along a special kind of path, allows it to be a state variable. Put another way, calculating changes in entropy from one state to another, we must construct ...
Thermodynamics Temperature Scales Thermal Expansion and Stress
... energy source in the tropics. Surface water at 29oC could act as a hot reservoir, and deep water at 3.0oC could serve as a cold reservoir. Ammonia gas could be a working fluid in a heat engine that runs between the two thermal reservoirs. What is the maximum efficiency of such an engine? ...
... energy source in the tropics. Surface water at 29oC could act as a hot reservoir, and deep water at 3.0oC could serve as a cold reservoir. Ammonia gas could be a working fluid in a heat engine that runs between the two thermal reservoirs. What is the maximum efficiency of such an engine? ...
A non-equilibrium quantum thermodynamics approach to
... Project supervisors: Mauro Paternostro and Tchavdar Todorov Contacts: [email protected] and [email protected] State of the art and motivations Thermodynamics is one of the pillars of physical, chemical and biological sciences. It predicts and explains the occurrence and efficiency of complex ...
... Project supervisors: Mauro Paternostro and Tchavdar Todorov Contacts: [email protected] and [email protected] State of the art and motivations Thermodynamics is one of the pillars of physical, chemical and biological sciences. It predicts and explains the occurrence and efficiency of complex ...
15.1,2
... The system and its surroundings are separated by walls of some kind. Walls that permit heat to flow through them, such as those of the engine block, are called diathermal walls. Perfectly insulating walls that do not permit heat to flow between the system and its surroundings are called adiabatic wa ...
... The system and its surroundings are separated by walls of some kind. Walls that permit heat to flow through them, such as those of the engine block, are called diathermal walls. Perfectly insulating walls that do not permit heat to flow between the system and its surroundings are called adiabatic wa ...
FE Review Common Pitfalls in Thermodynamics
... 7. Integrals of PdV—Boundary work done by a working fluid within a system is given as the integral of PdV. Before one can successfully perform this integral, the process of the working fluid within the system must be known and the pressure as a function of volume must be determined. This integral is ...
... 7. Integrals of PdV—Boundary work done by a working fluid within a system is given as the integral of PdV. Before one can successfully perform this integral, the process of the working fluid within the system must be known and the pressure as a function of volume must be determined. This integral is ...
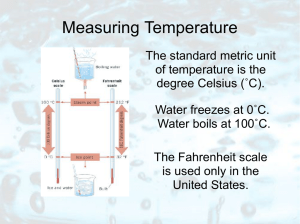
Measuring Temperature
... the heat added to a system will show up as an increase in the system's internal energy plus any external work done by the system. A good example of this would be an automobile engine. ...
... the heat added to a system will show up as an increase in the system's internal energy plus any external work done by the system. A good example of this would be an automobile engine. ...
Chapter 12 Laws of Thermodynamics
... If you shuffle them, they get disorganized. Shuffle again, they get more disorganized. The entropy of the deck of cards is increased. Easy to disorganize things, but it takes considerable effort and lots of luck to organize ...
... If you shuffle them, they get disorganized. Shuffle again, they get more disorganized. The entropy of the deck of cards is increased. Easy to disorganize things, but it takes considerable effort and lots of luck to organize ...
thermodynamics - CHM152-SP10
... of all reactants, initially present in their standard states, to all products in their standard states. DG is the free energy change for other ...
... of all reactants, initially present in their standard states, to all products in their standard states. DG is the free energy change for other ...
Chapter 15 Notes - Valdosta State University
... wall. One that does not is said to be adiabatic. The state of a system is described by specifying factors that affect the internal energy of the system. In the case of a gas this would be temperature, pressure, volume, and mass. There are three laws of thermodynamics. The first one is called the Zer ...
... wall. One that does not is said to be adiabatic. The state of a system is described by specifying factors that affect the internal energy of the system. In the case of a gas this would be temperature, pressure, volume, and mass. There are three laws of thermodynamics. The first one is called the Zer ...
Heat and Thermodynamics
... The internal energy U might be thought of as the energy required to create a system in the absence of changes in temperature or volume. But if the process changes the volume, as in a chemical reaction which produces a gaseous product, then work must be done to produce the change in volume. For a co ...
... The internal energy U might be thought of as the energy required to create a system in the absence of changes in temperature or volume. But if the process changes the volume, as in a chemical reaction which produces a gaseous product, then work must be done to produce the change in volume. For a co ...
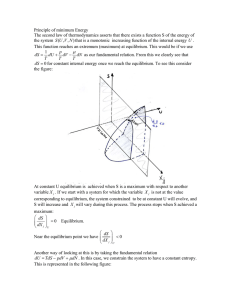
Principle of minimum Energy The second law of thermodynamics
... dU = TdS − pdV + μ dN . In this case, we constrain the system to have a constant entropy. This is represented in the following figure: ...
... dU = TdS − pdV + μ dN . In this case, we constrain the system to have a constant entropy. This is represented in the following figure: ...
Chapter 1: The first law of thermodynamics
... and volume we say that the quantity is a function of state. Therefore, for an ideal gas in equilibrium, the system’s temperature is a function of state ( θ = F ( P,V ) ). A quantity, dG, is said to be an exact differential if it only depends on the difference in the function of state between two clo ...
... and volume we say that the quantity is a function of state. Therefore, for an ideal gas in equilibrium, the system’s temperature is a function of state ( θ = F ( P,V ) ). A quantity, dG, is said to be an exact differential if it only depends on the difference in the function of state between two clo ...
Lecture 1 1 Overview
... Consider a fluid of C independent components and P coexisting phases. How many variables do we have? It is sufficient to specify a quantity for each component and the temperature and pressure in each phase, i.e., T α , P α , nα1 , . . . , nαC . Note that specifying nα1 , . . . , nαC is the same as s ...
... Consider a fluid of C independent components and P coexisting phases. How many variables do we have? It is sufficient to specify a quantity for each component and the temperature and pressure in each phase, i.e., T α , P α , nα1 , . . . , nαC . Note that specifying nα1 , . . . , nαC is the same as s ...