States of matter - Tennessee State University
... The change in entropy between two equilibrium states is given by the heat transferred, dQr, in a quasi-static process leading from the initial to the final state divided by the absolute temperature, T, of the system ...
... The change in entropy between two equilibrium states is given by the heat transferred, dQr, in a quasi-static process leading from the initial to the final state divided by the absolute temperature, T, of the system ...
solutions
... • Zeroth: If two systems are both in thermal equilibrium with a third then they are in thermal equilibrium with each other. • First: The increase in internal energy of a closed system is equal to the heat supplied to the system minus work done by it. • Second: The entropy of any isolated system neve ...
... • Zeroth: If two systems are both in thermal equilibrium with a third then they are in thermal equilibrium with each other. • First: The increase in internal energy of a closed system is equal to the heat supplied to the system minus work done by it. • Second: The entropy of any isolated system neve ...
0.1 Minimum Principles and Thermodynamic Potentials
... The minimum principle for G then states that for all states at a fixed T and P , the equilibrium state is that for which G is a minimum. The proof is very similar to that for A: the second law states that ∆Q ≤ T ∆S or 0 ≥ T ∆S + ∆U + P ∆V , if P is held fixed. But dG = dU − T dS + P dV , so dG ≤ 0 i ...
... The minimum principle for G then states that for all states at a fixed T and P , the equilibrium state is that for which G is a minimum. The proof is very similar to that for A: the second law states that ∆Q ≤ T ∆S or 0 ≥ T ∆S + ∆U + P ∆V , if P is held fixed. But dG = dU − T dS + P dV , so dG ≤ 0 i ...
15.3 The First Law of Thermodynamics
... can have a greater efficiency than a reversible engine operating between the same temperatures. Furthermore, all reversible engines operating between the same temperatures have the same efficiency. ...
... can have a greater efficiency than a reversible engine operating between the same temperatures. Furthermore, all reversible engines operating between the same temperatures have the same efficiency. ...
CARNOT CYCLE i) substance starts at with temperature T2
... 1. There exists a STATE VARIABLE for any substance called the ENTROPY. 2. Entropy (S) may change by one of two ways; the substance may contact a thermal reservoir (heat source), or S may change due to “internal” changes in the substance. dse (externally-forced changes in S) dsi (internally-forced ...
... 1. There exists a STATE VARIABLE for any substance called the ENTROPY. 2. Entropy (S) may change by one of two ways; the substance may contact a thermal reservoir (heat source), or S may change due to “internal” changes in the substance. dse (externally-forced changes in S) dsi (internally-forced ...
Equilibrium Thermodynamics
... revolution.) Thermodynamics is so general that it would work even if matter did not consist of atoms and molecules! - The drawback of its generality is that there are a lot of abstract concepts to learn and understand before we can get to interesting applications. As usual, more general theories ar ...
... revolution.) Thermodynamics is so general that it would work even if matter did not consist of atoms and molecules! - The drawback of its generality is that there are a lot of abstract concepts to learn and understand before we can get to interesting applications. As usual, more general theories ar ...
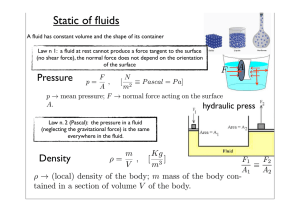
Static of fluids
... Second principle of thermodynamics It is impossible to realize a cyclic machine whose only result is to transform in work all the heat absorbed by a homogeneous source (Kelvin-Planck formulation). ...
... Second principle of thermodynamics It is impossible to realize a cyclic machine whose only result is to transform in work all the heat absorbed by a homogeneous source (Kelvin-Planck formulation). ...
Thermodynamics
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
Thermodynamics and the aims of statistical mechanics
... the scale; and (ii) the zero point. For example, inspired by Boyle’s Law that for gases at fixed temperature, p and V are inversely proportional, we might define a scale determined by the change of volume at constant pressure (or vice versa). (It turns out that as p → 0, different gases give the sam ...
... the scale; and (ii) the zero point. For example, inspired by Boyle’s Law that for gases at fixed temperature, p and V are inversely proportional, we might define a scale determined by the change of volume at constant pressure (or vice versa). (It turns out that as p → 0, different gases give the sam ...
幻灯片 1
... a) A simple engine uses a perfect gas as the working fluid in a piston-cylinder system. The gas is first heated at constant pressure from state 1 to state 2, then cooled at constant volume to state 3 where T3=T1, and then cooled at constant temperature, thereby returning to state 1. Derive expres ...
... a) A simple engine uses a perfect gas as the working fluid in a piston-cylinder system. The gas is first heated at constant pressure from state 1 to state 2, then cooled at constant volume to state 3 where T3=T1, and then cooled at constant temperature, thereby returning to state 1. Derive expres ...
ME 7280 Statistical Thermodynamics
... constituents of which systems are comprised. We will begin by using elements of probability to develop the properties if systems composed of non-interacting, independent particles. Later in the course we will introduce the theory of ensembles to find the probability that systems of the ensemble are ...
... constituents of which systems are comprised. We will begin by using elements of probability to develop the properties if systems composed of non-interacting, independent particles. Later in the course we will introduce the theory of ensembles to find the probability that systems of the ensemble are ...
Chapter 1 Thermodynamics
... the Second Law are equivalent. Solution: Assume that Kelvin statement is false ∆ Extract Q of heat from a reservoir at temperature T2 and convert it entirely to work W = Q. Then convert this work back into heat Q = W and transfer it to reservoir at temperature T1 > T2 ∆Clausius statement is false. A ...
... the Second Law are equivalent. Solution: Assume that Kelvin statement is false ∆ Extract Q of heat from a reservoir at temperature T2 and convert it entirely to work W = Q. Then convert this work back into heat Q = W and transfer it to reservoir at temperature T1 > T2 ∆Clausius statement is false. A ...
Concept of Energy
... Since the internal energy is fixed when one specifies the entropy and the volume, this relation is valid even if the change from one state of thermal equilibrium to another with infinitesimally larger entropy and volume happens in a non-quasistatic way (so during this change the system may be very ...
... Since the internal energy is fixed when one specifies the entropy and the volume, this relation is valid even if the change from one state of thermal equilibrium to another with infinitesimally larger entropy and volume happens in a non-quasistatic way (so during this change the system may be very ...
File - SPHS Devil Physics
... the second law of thermodynamics, this area of physics demonstrates the collaboration and testing involved in confirming abstract notions such as this. ...
... the second law of thermodynamics, this area of physics demonstrates the collaboration and testing involved in confirming abstract notions such as this. ...
Topic 2 The first law of thermodynamics
... State function is a property that is independent of how a sample is prepared, completely differential ,single valued Properties that relate to the preparation of the state are called path functions Question: T, P, V, ρ, Vm…… W, Q ...
... State function is a property that is independent of how a sample is prepared, completely differential ,single valued Properties that relate to the preparation of the state are called path functions Question: T, P, V, ρ, Vm…… W, Q ...
System stability
... should be the highest (but it is constant in this non-relativistic analysis), the moment of inertia should be the highest (i.e. deformable systems tend to rotate around the principal axis of inertia), and the position in the gravity field should be the lowest. As a consequence, at equilibrium, the ...
... should be the highest (but it is constant in this non-relativistic analysis), the moment of inertia should be the highest (i.e. deformable systems tend to rotate around the principal axis of inertia), and the position in the gravity field should be the lowest. As a consequence, at equilibrium, the ...
Chapter 6 ()
... or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed to discuss the waves in ...
... or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed to discuss the waves in ...
Entropy, Carnot Engine and Thermoelectric Effect
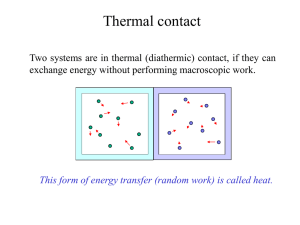
... Thermal Equilibrium : Two systems placed in contact with each other are said to be in thermal equilibrium if no net transfer of heat takes place between them. Mechanical Equilibrium : Two mechanically connected systems are said to be mechanical equilibrium if they exert equal and opposite mechanical ...
... Thermal Equilibrium : Two systems placed in contact with each other are said to be in thermal equilibrium if no net transfer of heat takes place between them. Mechanical Equilibrium : Two mechanically connected systems are said to be mechanical equilibrium if they exert equal and opposite mechanical ...
Objectives Recognize that a system can absorb or release energy
... o A steady decrease in the car’s total mechanical energy occurs because of work being done against the _________________________ between the car’s axles and its bearings and between the car’s wheels and the coaster track. o If the internal energy for the roller coaster (the system) and the energy di ...
... o A steady decrease in the car’s total mechanical energy occurs because of work being done against the _________________________ between the car’s axles and its bearings and between the car’s wheels and the coaster track. o If the internal energy for the roller coaster (the system) and the energy di ...
Chapter 6 Thermodynamics and the Equations of Motion
... waves in the atmosphere or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed ...
... waves in the atmosphere or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included more physics than we need, for example the compressibility of water is not needed ...
Thermodynamics - myersparkphysics
... equation for WORK is often misunderstood. Since work done BY a gas has a positive volume change we must understand that the gas itself is USING UP ENERGY or in other words, it is losing energy, thus the negative ...
... equation for WORK is often misunderstood. Since work done BY a gas has a positive volume change we must understand that the gas itself is USING UP ENERGY or in other words, it is losing energy, thus the negative ...
Thermodynamics
... A particular engine has a power output of 5000 W and an efficiency of 25%. If the engine expels 8000 J of heat in each cycle, find (a) the heat absorbed in each cycle and (b) the time for each cycle ...
... A particular engine has a power output of 5000 W and an efficiency of 25%. If the engine expels 8000 J of heat in each cycle, find (a) the heat absorbed in each cycle and (b) the time for each cycle ...
Fundamentals of chemical thermodynamics and bioenergetics
... The secret of snowmaking is in the equation ΔU = Q + W. A snowmaking machine contains a mixture of compressed air and water vapor at about 20 atm. Because of the large difference in pressure between the tank and the outside atmosphere, when the mixture is sprayed into the atmosphere it expands so ra ...
... The secret of snowmaking is in the equation ΔU = Q + W. A snowmaking machine contains a mixture of compressed air and water vapor at about 20 atm. Because of the large difference in pressure between the tank and the outside atmosphere, when the mixture is sprayed into the atmosphere it expands so ra ...
introduction
... connected the wrong electrode as cathode. This is equivalent to reversing equation (1). When equation (1) is reversed, the measured cell potential difference becomes Eocell = - 1.10 V. The absolute value of ∆Eocell is the same in both cases, but the sign is different. The sign of ∆Eocell is positive ...
... connected the wrong electrode as cathode. This is equivalent to reversing equation (1). When equation (1) is reversed, the measured cell potential difference becomes Eocell = - 1.10 V. The absolute value of ∆Eocell is the same in both cases, but the sign is different. The sign of ∆Eocell is positive ...