* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CARNOT CYCLE i) substance starts at with temperature T2
R-value (insulation) wikipedia , lookup
Conservation of energy wikipedia , lookup
Equipartition theorem wikipedia , lookup
Thermal radiation wikipedia , lookup
Heat capacity wikipedia , lookup
Thermal expansion wikipedia , lookup
Calorimetry wikipedia , lookup
Van der Waals equation wikipedia , lookup
Temperature wikipedia , lookup
Heat equation wikipedia , lookup
Countercurrent exchange wikipedia , lookup
State of matter wikipedia , lookup
Heat transfer wikipedia , lookup
Internal energy wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Thermal conduction wikipedia , lookup
Equation of state wikipedia , lookup
Heat transfer physics wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Maximum entropy thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
History of thermodynamics wikipedia , lookup
Gibbs free energy wikipedia , lookup
Adiabatic process wikipedia , lookup
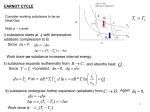
CARNOT CYCLE Consider working substance to be an Ideal Gas Note p – v axes i) substance starts at with temperature T2, adiabatic compression to B. Since Work done on substance increases internal energy. ii) substance expands isothermally from Since =constant, and absorbs heat . P dv = iii) substance undergoes further expansion (adiabatic) from Work done is Again 1 iv) substance returns to original point by undergoing an isothermal compression from D A , and the heat exhausted is equal to Q2. Since du=0 for this isothermal process, dq = dw: or Since the working substance returns to its original state, the work done by the substance is equal to the net heat absorbed, Q1-Q2 Work done = Q1-Q2 (area inside the “rectangle”) This can be readily verified by summing the individual work terms. This can be easily verified by adding up the individual terms. Efficiency of the carnot cycle is Q: How can efficiency be increased? 2 Only by transferring heat from a warm body to a cold body can heat be converted to work in a cyclic process. For a CARNOT CYCLE Proof: It can be shown that = constant for adiabatic process Adiabatic legs For A B For C D Isothermal legs For B C For D A Combining 4 equations We already know that 3 maximum (Carnot) efficiency of a power cycle Therefore So η = 1− Q2 T T = 1− 2 = 1− cold Q1 T1 Twarm • The maximum efficiency of an energy cycle is given by the Carnot efficiency, which can be expressed as: η€ = 1− Tc Th If we consider the temperature difference between the equator and the poles: Tpole € (Tc) Tequator (Th) We note that Th varies little throughout the year (estimate as ~301 K), compared with Tc Winter hemisphere: estimate Tc ~ 263 K η = 1− Tc 263 = 1− ≈ 13% Th 301 Summer hemisphere: estimate Tc ~ 283 K € η = 1− Tc 283 = 1− ≈ 6% Th 301 --> More of the absorbed energy is converted into “work” in the winter hemisphere 4 The hurricane as an energy cycle Consider a hurricane as a Carnot cycle: http://apollo.lsc.vsc.edu/classes/met130/notes/chapter15/vertical_circ.html 3 Toutflow 4 2 1 Tsea surface Analyze each leg of the cycle: 1: absorption of energy from ocean, ~isothermal expansion 2: convection in the eyewall, ~adiabatic expansion 3: upper level outflow, ~ isothermal compression (reject heat via radiation) 4: sinking branch, ~adiabatic compression If we estimate SST~300 K, and outflow T~200 K, η = 1− Tc 200 = 1− ≈ 30% Th 300 U Hawaii class notes: Latent heat released when water vapor condenses in clouds is the key. Hurricanes are giant engines that convert heat into wind energy. Consider a rain rate of 2 inches per day over an area of 300 mi radius: Over a 7 day lifecycle, the energy released is equal to 50,000 1 MT nuclear explosions, equivalent to the total explosive yield of the nuclear arsenals of the US and USSR at the height of the Cold War! www.soest.hawaii.edu/MET/Faculty/businger/.../18cHurricanes1.pdf € Our next state function: ENTROPY (consequence of Second Law) There exists a function called entropy S, of the extensive variables of a system, defined for all equilibrium states, such that the values assumed by the extensive variables are those that maximize S (at equilibrium) From the viewpoint of classical thermodynamics, entropy is defined as where in this case heat is added to a substance undergoing a reversible transformation. Clausius (1822-1888) stated that the cyclic integral of δQ/T is always less than or equal to zero: ∫ δQ ≤0 T The cyclic integrals of work and of heat are not zero (engines). Entropy is a STATE VARIABLE. (We can make up an internally reversible path from A to B to compute change in entropy€ from the integral of δQ/T.) Since dq= Tds, the First Law can be written as, Tds = du + pdv € Even though we imagined a reversible process to substitute for dq, this equation applies to both reversible and irreversible transitions because they are state variables Generalized Statement of the Second Law • We need to generalize the definition of entropy since real systems are typically spontaneous and irreversible, moving from a state of non-equilibrium to a state of equilibrium. • Second law can be formulated as 4 postulates: 1. There exists a STATE VARIABLE for any substance called the ENTROPY. 2. Entropy (S) may change by one of two ways; the substance may contact a thermal reservoir (heat source), or S may change due to “internal” changes in the substance. dse (externally-forced changes in S) dsi (internally-forced changes in S) Examples: dse Heat flow into a substance causing molecules to rearrange dsi Mixing a gas, forcing molecules to change position. Total entropy change ds = dse + dsi 3. Changes in S due to external influences are given by where dq = heat imported or exported, while substance is in contact with a thermal reservoir at temperature • 4. For reversible transformations, For irreversible transformations, So reversible and irreversible processes are distinguished by entropy changes. For reversible processes, there are no entropy changes associated with internal processes, or they have ceased as the system undergoes only reversible changes, say via contact with a heat reservoir. Now consider the system + its surroundings ( = “universe”) • In a reversible process any heat flow between the system and surroundings must occur with no finite temperature difference between the system and surroundings. Otherwise the process would be irreversible UNIVERSE Since • • Therefore for reversible process. As expected and can be shown via a similar argument, Irreversible process In general Statement of 2nd Law! Calculations of Entropy Changes • For reversible processes, • For irreversible processes, • However since S is a state function, depends only upon endpoints. Hence can be found by devising a series of reversible transformations that are equivalent to the irreversible transformations. is not necessarily equal to 1. Reversible adiabatic process Hence , hence 2. Reversible phase change at constant T, p since constant where m=mass, L= latent heat 3. Reversible, isothermal process 4. Reversible change of state for an Ideal Gas From First Law: then Heating term Volume term 5. Constant-pressure heating (irreversible) For constant pressure: Hence for 6. Similarly, for constant-volume heating (irreversible) dq rev = cv dT + pdv pdv → 0 T2 ΔS = ∫ cv ln dT T1 ⎛ T2 ⎞ ΔS = cv ln⎜ ⎟ ⎝ T1 ⎠ More entropy calculations at end of notes “Thermodynamic Potentials” • The internal energy, when expressed as a function of S and V, is one of the so-called “thermodynamic potentials”. The differential of a thermodynamic potential gives the first and second laws of thermodynamics combined: du = dq − dw du = Tds − Pdv • • • • S and V are the “native variables” for U (although we can express U as a function of any two variables) Remember € enthalpy is: H = U + PV dh = Tds + vdP So S and P are the “native variables” for H. We define two more thermodynamic potentials that have “native variables” , respectively, of T and V and T and P. € Very useful for equilibrium calcs! Helmholtz & Gibbs Functions • Consider the following system/reservoir arrangement, Q system • -Q Total work done by the system, whether it be in a reversible or irreversible process is, First Law • reservoir Q is positive if it flows into the system (1) w is positive if done by the system dq=du+dw From principle of entropy, w=Q-(u2-u1) (2) A general expression that entropy must stay constant or increase • For the reservoir, heat flow out of reservoir • Therefore we have • • Note T= constant; reservoir has infinite heat capacity. From (3) we have the following inequality, • From First Law or Eq (1) above (substitute for Q) (3) Reversible process Irreversible process • For the irreversible case the work done is less than since some of the heat added to the system can go into changing the ‘internal’ entropy of the system. (4) • The difference in this energy between 2 equilibrium states at the same temperature is (5) • Hence change in F sets an upper limit to the work that can be done in any process between 2 equilibrium states at constant T. • F is often referred to as the ‘free energy’ since ∆F represents the maximum amount of work that can be done in a transformation at constant temperature. • Returning to the equation (6) • This equation is general and can be applied to any system, change of state, phase, or even a chemical reaction. • In general, work in processes we are considering consist of pdV work and other possible forms of work, e.g. work required to form a curve interface between 2 phases, frictional dissipation, etc. • Let represent pdV type work and work, so we now have represent other forms of (7) • If we consider a constant volume process, • Hence at constant T and V, Helmholtz energy sets an upper limit to the non- pdV work that can be done in such a transition. • For a reversible constant volume process • Now consider a situation where no pdV and no work is done, then • Hence for a process at constant volume, for which (and T=constant), F can only decrease or remain constant. A transition will not occur if . Wikipedia definition: du = Tds − Pdv The Helmholtz free energy is a thermodynamic potential which measures the “useful” work dh = Tds + vdP obtainable from a closed thermodynamic system at a f = u −Ts (by definition) constant temperature and volume. For such a system, the negative of the difference in the df = du −Tds − sdT Helmholtz energy is equal to the maximum amount of work extractable from a thermodynamic process in = −sdT − Pdv which temperature and volume are held constant. Under these conditions (constant T and v), it is Constant v constraint is not minimized at equilibrium. (8) always helpful for us … € • Next, consider a process that occurs at constant pressure. In this case the pdV work is • Then returning to the equation: • We have: (8) Now define the Gibbs Free Energy as, (dG = -SdT + VdP) Then for a transition between 2 states at the same T ,p: and substituting into (8) we have ∆G represents the total non- pdV work for a constant T,P process. This is of fundamental importance for cloud physics. quick look ahead to one application we’ll be discussing: • • • During nucleation, some energy is released when molecules move from the vapor to the “liquid” phase is the work (energy required) necessary to form the surface of the small liquid (or ice) embryo from the vapor phase: If So G can only decrease in a transition occurring at constant T, P. • If the hypothetical nucleus is too small (known as an unstable nucleus or "embryo"), the energy that would be released by forming its volume is not enough to create its surface Thermodynamic Potential • • • Given Consider a closed system which is one where no mass is allowed to cross the boundaries of the system. Taking total differentials, (1) (2) • Using First Law in the form: • We can substitute for (1) and (2) the dU term • Consider the following series of equations, • It is useful to identify the coefficients of the equations on the left hand side with partial derivatives of the variables on the right hand side. • For: • Therefore we have • Since • Therefore • Since • And • Since • And • Since P, V, T and S can be expressed as partial derivatives of U, F, G and H, the latter variables are referred to as Thermodynamic Potentials. Maxwell Relations • The “Gibbs relations” are the following equations that we have already seen: du = Tds − Pdv dh = Tds + vdP da = −sdT − Pdv dg = −sdT + vdP • We recognize that they are exact differentials and have the form € dz = Mdx + Ndy ⎛ ∂M ⎞ ⎛ ∂N ⎞ ⎜ ⎟ = ⎜ ⎟ ⎝ ∂y ⎠ x ⎝ ∂x ⎠ y • We can deduce the “Maxwell Relations”: ⎛ ∂€T ⎞ ⎛ ∂P ⎞ ⎜ ⎟ = −⎜ ⎟ ⎝ ∂v ⎠ s ⎝ ∂s ⎠ v ⎛ ∂T ⎞ ⎛ ∂v ⎞ ⎜ ⎟ = ⎜ ⎟ ⎝ ∂P ⎠ s ⎝ ∂s ⎠ P ⎛ ∂s ⎞ ⎛ ∂P ⎞ ⎜ ⎟ = ⎜ ⎟ ⎝ ∂v ⎠T ⎝ ∂T ⎠ v ⎛ ∂s ⎞ ⎛ ∂v ⎞ ⎜ ⎟ = −⎜ ⎟ ⎝ ∂P ⎠T ⎝ ∂T ⎠ P Can get unmeasurables in terms of measurables Stable and Unstable Equilibrium • • Stable Equilibrium: a state in which all irreversible processes have ceased, and the system is in a state of maximum entropy. Reversible transformations are possible (for which ). For state equilibrium, MAX ENTROPY • • a) This equilibrium condition is for an isolated system (no heat transfer, no mass transfer) A typical system we will deal with is NOT in thermal isolation, but rather in contact with a heat reservoir (e.g., as approximated by the atmosphere). For a transition at constant volume, in thermal contact with a reservoir at temperature T, the stable state is defined by, Helmholtz free energy is a minimum b) For a transition at constant pressure and temperature, (in contact with a thermal reservoir), stable state is, Gibbs free energy is a minimum Applying equilibrium criteria • We can now add moisture to the air parcel we’ve computed changes for – We could have added water to the “dry air mixture” and computed a new average molecular weight, etc., BUT we know that simple approach, assuming water stays as a vapor during the adiabatic ascent, is only valid over small regions – That is, we’ll need to consider phase changes for the water • First, we’ll figure out where vapor, liquid and ice are in equilibrium, and the energy changes associated with the transitions – Can calculate changes such as heat released so we can treat the energy changes in our parcel – Can also figure out if the change is favored (e.g., if the transition is allowed to occur thermodynamically, given specified conditions) LATENT HEATS: During a phase change, the pressure is constant and equal to the saturation pressure (which is a function of T only). Adding heat to a substance at constant pressure and resulting in a change in physical state, i.e. change in phase, is described by, Starting with First Law in the dq = dh = Ldm form dq = dh when dp = 0 where L is the latent heat (J kg-1) for the particular transition and m is mass transferred between phases. The heat absorbed goes into changing the molecular configuration/structure. For water substance: € Heat of fusion L is an energy change to go from one phase to another Heat of condensation Heat of sublimation SOLID Lf LIQUID Lc VAPOR 26 LS Thermodynamics of Moist Air • Phase transitions: what are the equilibrium conditions for a mixed-phase system, e.g. a mixture of water and water vapor? • Consider a system consisting of liquid water and H2O(v) undergoing a change in state, vapor vapor liquid liquid ″ denotes liquid phase ″′ denotes vapor phase • Define • Hence • Since both states are stable equilibrium states, G1=G2, and therefore n1ʹ′ʹ′gʹ′ʹ′ + n1ʹ′ʹ′gʹ′ ʹ′ʹ′ʹ′ = n ʹ′2ʹ′gʹ′ʹ′ + n ʹ′2ʹ′ʹ′gʹ′ʹ′ʹ′ But since the system is closed ( n1ʹ′ʹ′ − n ʹ′2ʹ′) gʹ′ʹ′ = ( n ʹ′2ʹ′ʹ′ − n1ʹ′ʹ′ʹ′) gʹ′ʹ′ʹ′ Therefore • • n1ʹ′ʹ′ + n1ʹ′ʹ′ʹ′= n ʹ′2ʹ′ + n ʹ′2ʹ′ʹ′ n ʹ′2ʹ′ = n1ʹ′ʹ′ + n1ʹ′ʹ′ʹ′− n ʹ′2ʹ′ʹ′ • • • At equilibrium, the specific Gibbs free energy is the same for both phases At the triple point, Lets look at g between 2 phases in more detail: vapor c g liquid b,e f d a T • From earlier discussions, • Where s′″ is the specific entropy of the vapor phase. Hence the slope of gives the specific entropy. Likewise for the liquid phase • • Note the slopes are <0, since larger values of g correspond to lower T’s. The difference between entropies is • Since vapor liquid vs. T Clausius-Clapeyron Equation • This equation is very important since it describes the variation in pressure with temperature, for a system consisting of two phases in equilibrium. For the case of a liquid/vapor or vapor/solid system, this “pressure” is the saturation vapor pressure. vaporʹ′ʹ′ʹ′ liquid ʹ′ʹ′ • For a system in equilibrium, • € Suppose the system undergoes a small change to a new pressure and temperature, p+dp, T+dT such that the new state is also a stable state. Each phase changes by amounts. Then for each phase: • Since the new state is also an equilibrium state, • • Or, s, v are specific quantities! CLAUSIUS-CLAPEYRON EQUATION • Since =latent heat for vapor-liquid interface For vapor-liquid system • • • For vapor-liquid system For vapor-ice system Geometrical Interpretation SOLID LIQUID S-L L-V P Triple point Critical point S-V VAPOR T gives the slope of the equilibrium line between the 2 phases involved. (on P-V-T diagram.) • Looking again at the Clausius-Clapeyron Equation we have three interfaces, vaporliquid solidvapor liquidsolid • 1. Since always, the slope of the equilibrium line between the 2 phases involved is determined by the difference in specific volumes. vaporliquid vaporsolid ] ALWAYS For all materials! 2. For substance that expands upon freezing (like water). Hence P L S Triple V T substance contracts upon freezing. MOST SUBSTANCES! • Integrate the Clausius-Clapeyron Eqn/ to get es as a function of T. For a vapor/liquid system, • Assume GAS LAW • Therefore after integration More entropy calculations 35 Rapid expansion 7. Irreversible change of state of an Ideal Gas Let n moles of an ideal gas of irreversibly change state to This irreversible process can be broken into 2 reversible processes: 1.) A slow expansion to at constant -put gas in a frictionless piston-cylinder, place in constant temperature bath; slowly move piston out 2.) Hold gas at , heat gas to temperature and pressure Hence Constant-volume heating Isothermal expansion 8. Irreversible phase change Let 10g of supercooled water at -10°C change to ice at -10°C. Consider constant pressure. This process is IRREVERSIBLE e.g. if the ice is slowly heated, it will not become water at -10°C. Water at -10°C IRREVERSIBLE Ice at -10°C 1 Water at 0°C 3 2 Ice at 0°C Find equivalent reversible path and compute entropy change 1.) Isobaric heating of water 2.) Phase change 3.) Isobaric cooling of ice More entropy calculations • Calculate the change in entropy when 5g of water at 0°C are raised to 100°C and converted into vapor (steam) at that temperature. 1. Raise temperature of H2O(l) from 0°C to 100°C Let Specific heat of water 2. Convert to vapor (steam) at 100°C • Find for the melting of 5g of ice at 0°C at 1 atm. This process is reversible since both ice and liquid states are possible at 0°C. (Equilibrium states!) • For freezing of liquid water, and Why the specific signs for melting vs. freezing? • Let n moles of a perfect gas undergo an adiabatic free expansion into a vacuum (this is Joule’s experiment). – Express ∆S in terms of the initial and final temperature and volume. – Calculate ∆S if • The initial state is and the final state is Although the process is adiabatic, (dq=0), entropy change is non-zero because this process is irreversible. Entropy of course changes (and increases) since the molecules changed their configuration expanding into the vacuum. Hence With This is the so-called Entropy of Mixing.