Questions - TTU Physics
... 2. PLEASE do not write on the exam sheets, there will not be room! Use other paper!! 3. PLEASE show all of your work, writing down at least the essential steps in the solution of a problem. Partial credit will be liberal, provided that the essential work is shown. Organized work, in a logical, easy ...
... 2. PLEASE do not write on the exam sheets, there will not be room! Use other paper!! 3. PLEASE show all of your work, writing down at least the essential steps in the solution of a problem. Partial credit will be liberal, provided that the essential work is shown. Organized work, in a logical, easy ...
More Thermodynamics
... of work the gas does, or . Since the gas expands freely (the volume change of the system is zero), we know that no work will be done, so W=0. Since both chambers are insulated, we also know that Q=0. Thus, the internal energy of the gas does not change during this process. More Thermodynamics ...
... of work the gas does, or . Since the gas expands freely (the volume change of the system is zero), we know that no work will be done, so W=0. Since both chambers are insulated, we also know that Q=0. Thus, the internal energy of the gas does not change during this process. More Thermodynamics ...
Chapter 15
... hot body to a cold one entropy increases and order goes to disorder the separation of hot/cold objects can serve as high/low temperature regions for a heat engine and be used to obtain useful work when the two objects reach the same temperature no work can be obtained ...
... hot body to a cold one entropy increases and order goes to disorder the separation of hot/cold objects can serve as high/low temperature regions for a heat engine and be used to obtain useful work when the two objects reach the same temperature no work can be obtained ...
heat engine
... Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
... Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
H92-Modeling Convection in the Mantle Analysis
... 5. Based on what you have seen in the jar, what effect do you think convection in the hot mantle might have on the earth’s plates? What observations of the fluid inside the jar support your explanation? ...
... 5. Based on what you have seen in the jar, what effect do you think convection in the hot mantle might have on the earth’s plates? What observations of the fluid inside the jar support your explanation? ...
Thermodynamic system
... • Temperature quantifies the kinetic energy of microscopic thermal motion (recall the equipartition theorem) • Heat is energy transfer between two systems (apart from work) • Temperature is a state variable, heat is not (depends on process path) • Isothermal vs. adiabatic process: – if system is the ...
... • Temperature quantifies the kinetic energy of microscopic thermal motion (recall the equipartition theorem) • Heat is energy transfer between two systems (apart from work) • Temperature is a state variable, heat is not (depends on process path) • Isothermal vs. adiabatic process: – if system is the ...
P - School of Chemical Sciences
... Why Thermodynamics? The macroscopic description of a system of ~1023 particles may involve only a few variables! “Simple systems”: Macroscopically homogeneous, isotropic, uncharged, large enough that surface effects can be neglected, not acted upon by electric, magnetic, or gravitational fields. ...
... Why Thermodynamics? The macroscopic description of a system of ~1023 particles may involve only a few variables! “Simple systems”: Macroscopically homogeneous, isotropic, uncharged, large enough that surface effects can be neglected, not acted upon by electric, magnetic, or gravitational fields. ...
pdf 728k
... and what criteria govern these magnitudes? The answers to these questions require an examination of the nature of processes which leads to the development of the 2nd law The 1st and the 2nd laws lay the foundation of describing the thermodynamic behaviour of matter. ...
... and what criteria govern these magnitudes? The answers to these questions require an examination of the nature of processes which leads to the development of the 2nd law The 1st and the 2nd laws lay the foundation of describing the thermodynamic behaviour of matter. ...
THERMODYNAMIC REVIEW PROBLEMS: ME 435 Fall 2004
... 1. Air undergoes a process from an initial state where p1 = 14.0 lbf/in2, V1 = 500 in3 to a final state where p2 = 60 lbf/in2, V2 = 160 in3,. The relationship between pressure and volume during the process is pVn = constant. Determine the value of the constant n and calculate the work in BTU. 2. Ste ...
... 1. Air undergoes a process from an initial state where p1 = 14.0 lbf/in2, V1 = 500 in3 to a final state where p2 = 60 lbf/in2, V2 = 160 in3,. The relationship between pressure and volume during the process is pVn = constant. Determine the value of the constant n and calculate the work in BTU. 2. Ste ...
Example 2 - The Graduate School | UNC Charlotte
... and stability for specified thermodynamic and solvent conditions in fast computing times for high throughput applications. ...
... and stability for specified thermodynamic and solvent conditions in fast computing times for high throughput applications. ...
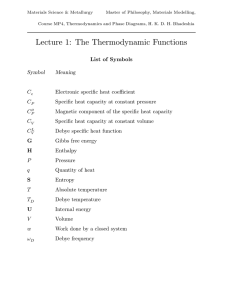
Thermodynamic functions - Phase Transformations Group
... no matter how long one waits”. For example, there will be no tendency for diffusion to occur between two phases which are in equilibrium even though they may have different chemical compositions. An equilibrium phase diagram is vital in the design of materials. ...
... no matter how long one waits”. For example, there will be no tendency for diffusion to occur between two phases which are in equilibrium even though they may have different chemical compositions. An equilibrium phase diagram is vital in the design of materials. ...
Thermodynamics
... Thermal energy is the energy a substance or system has due to its temperature, i.e., the energy of moving or vibrating molecules. Thermodynamics involves measuring this energy, which can be "exceedingly complicated," according to David McKee, a professor of physics at Missouri Southern State Univers ...
... Thermal energy is the energy a substance or system has due to its temperature, i.e., the energy of moving or vibrating molecules. Thermodynamics involves measuring this energy, which can be "exceedingly complicated," according to David McKee, a professor of physics at Missouri Southern State Univers ...
U3MEA02 Basic Engineering Thermodynamics
... SECOND LAW OF THERMODYNAMICS • Kelvin statement: It is impossible, by means of inanimate material agency, to derive mechanical effect from any portion of matter by cooling it below the temperature of the coldest of the surrounding objects. • Clausius statement: Heat can never pass from a colder to ...
... SECOND LAW OF THERMODYNAMICS • Kelvin statement: It is impossible, by means of inanimate material agency, to derive mechanical effect from any portion of matter by cooling it below the temperature of the coldest of the surrounding objects. • Clausius statement: Heat can never pass from a colder to ...
LECTURE 5 Temperature Scales The equation of state of any
... value of the entropy of a state can be defined because the third law of thermodynamics tells us that as T → 0, the entropy S approaches a definite value So which is usually 0. A simple example is a system of N magnetic atoms, each with spin 1/2. If this system is known to be ferromagnetic at suffici ...
... value of the entropy of a state can be defined because the third law of thermodynamics tells us that as T → 0, the entropy S approaches a definite value So which is usually 0. A simple example is a system of N magnetic atoms, each with spin 1/2. If this system is known to be ferromagnetic at suffici ...
Lecture 3: 09.14.05 The first law of thermodynamics
... Work and heat are not state functions; they are path dependent- what does this mean? In most physical situations, we are concerned with a quantity of heat or work transferred into or out of a material, which causes a change from one state of the material to another. Path dependence implies that the ...
... Work and heat are not state functions; they are path dependent- what does this mean? In most physical situations, we are concerned with a quantity of heat or work transferred into or out of a material, which causes a change from one state of the material to another. Path dependence implies that the ...
Chapter 5 auxiliary functions
... If again the system is a fixed quantity o an ideal gas , the equation of statue indicates that the enthalpy o an ideal as is independent of its pressure . ...
... If again the system is a fixed quantity o an ideal gas , the equation of statue indicates that the enthalpy o an ideal as is independent of its pressure . ...
Lecture 6 Free Energy
... Force in Mechanics In classical mechanics, potential energy U of an object can be defined in terms of the work required to move the object from A to B with no net change in kinetic energy. ...
... Force in Mechanics In classical mechanics, potential energy U of an object can be defined in terms of the work required to move the object from A to B with no net change in kinetic energy. ...
grand canonical partition function
... 2 Grand canonical partition function o o2.1 Definition 2.2 Relation to thermoynamic variables o2.3 Discussion ...
... 2 Grand canonical partition function o o2.1 Definition 2.2 Relation to thermoynamic variables o2.3 Discussion ...
Thermodynamics
... Zeroth Law of Thermodynamics – if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other Allows for a definition of temperature (two objects have the same temperature when they are in thermal equilibirum) ...
... Zeroth Law of Thermodynamics – if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other Allows for a definition of temperature (two objects have the same temperature when they are in thermal equilibirum) ...
Examples Paper 2 (1-2)
... enclosing control volumes, even though it is not the only, and arguably not always the best, way. Recall that: “a control volume is a region in space separated from its surroundings by a real or imaginary boundary, the control surface, across which mass may pass”. In flow processes, and given the es ...
... enclosing control volumes, even though it is not the only, and arguably not always the best, way. Recall that: “a control volume is a region in space separated from its surroundings by a real or imaginary boundary, the control surface, across which mass may pass”. In flow processes, and given the es ...
Thermodynamics
... W = PΔV 1st Law of Thermodynamics: ΔU = Q – W Define: Adiabatic, isothermal, isobaric & isochoric and show these on a P-V diagram Irreversibility & disorder Entropy is a measure of disorder State 2nd Law of Thermodynamics Heat engine efficiency, η = W/Qh Carnot Engine Energy Degradation ...
... W = PΔV 1st Law of Thermodynamics: ΔU = Q – W Define: Adiabatic, isothermal, isobaric & isochoric and show these on a P-V diagram Irreversibility & disorder Entropy is a measure of disorder State 2nd Law of Thermodynamics Heat engine efficiency, η = W/Qh Carnot Engine Energy Degradation ...
Slide 1 - KaiserScience
... consequence of the second law, and is unavoidable; it can be reduced only by reducing the amount of energy we use. ...
... consequence of the second law, and is unavoidable; it can be reduced only by reducing the amount of energy we use. ...
Slide 1
... consequence of the second law, and is unavoidable; it can be reduced only by reducing the amount of energy we use. ...
... consequence of the second law, and is unavoidable; it can be reduced only by reducing the amount of energy we use. ...