* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 15
Equipartition theorem wikipedia , lookup
Heat exchanger wikipedia , lookup
Calorimetry wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Heat capacity wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Thermoregulation wikipedia , lookup
Thermal radiation wikipedia , lookup
Temperature wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Heat equation wikipedia , lookup
Conservation of energy wikipedia , lookup
R-value (insulation) wikipedia , lookup
Internal energy wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat transfer wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermal conduction wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
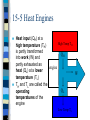
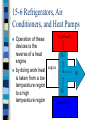
Chapter 15 The Laws of Thermodynamics Thermodynamics The study of the processes in which energy is transferred as heat and as work heat--transfer of energy due to a temperature difference work--transfer of energy not due to a temperature difference Thermodynamics System--any object or set of objects that we wish to consider closed system--a system for which no mass enters or leaves (but energy can) open system--mass as well as energy can be exchanged with environment isolated closed system--neither mass or energy passes across system boundary 15.1 The First Law of Thermodynamics based on the law of conservation of energy the change in the internal energy of a closed system, ( U), will be equal to heat added to system minus work done by the system U=Q-W 15.1 The First Law of Thermodynamics U=Q-W where: U, Internal energy (the total of all energy of all the molecules of a system) U = positive when internal energy of system increases U = negative when internal energy of system decreases 15.1 The First Law of Thermodynamics U=Q-W where: Q = heat heat added to the system is positive heat lost from the system is negative 15.1 The First Law of Thermodynamics U=Q-W where: W = work work done by the system is positive work done to the system is negative 15.1 The First Law of Thermodynamics For isolated systems: U = 0 so 0 = Q - W and Q=W=0 so no work is done by an isolated system 15.1 The First Law of Thermodynamics The amount of internal energy is a property of a system; heat and work is not a system doesn’t have a given amount of heat or work; rather they are added or removed form the system by the change of state of the system so heat and work are part of a thermodynamic process that can change a system from one state to another not a characteristic of the system such as P, V, T, n, or U. 15-2 The 1st Law of Thermodynamics Applied to Some Simple Systems Simples Processes: Isothermic Process Adiabatic Process Isobaric Process Isochoric (iosvolumetric) Process Isothermic Process Idealized process carried out at constant temperature for ideal gases; PV=nRT so PV= constant Isothermic Process Example: ideal gas in a cylinder fitted with a movable piston gas is in contact with a heat reservoir (body of mass so large that there is no significant T to it when heat is exchange with it) assume that compression or expansion is done slowly enough that all gas stays at equilibrium at same constant T Isothermic Process If heat (Q = +) is added to the system & T=constant then the gas must expand and do work on the surroundings (W = +) Work done by system Heat added to system Isothermic Process Since T is constant there is no U (U=3/2nRT--Ch-14) so U = Q - W or Q = W so in an isothermic process work done by the system is equal to the heat added to the system Work done by system Heat added to system Adiabatic Process Process in which no heat is allowed to flow into or out of the system Q=0 can occur if process happens so quickly that heat has no time to flow into or out of system Adiabatic Process Since Q = 0 then U = - W U decreases (U = -) if gas expands and does work by the system (W = +) temperature also decreases as well Temperature decreases (U=3/2nRT) Work by the system decrease in internal energy Adiabatic Process If gas contracts work is done on the system so U and T increases diesel engines compress fuel mixture by a factor of 15+ so it ignites without spark plugs Temperature increases Work on the system increase in internal energy Adiabatic Process Example: hold a rubber band loosely with hands gauge temperature by holding to lips stretch band suddenly quickly touch to lips temperature increases, WHY? Isobaric Process Pressure is kept constant since P = constant, P = F/A so F = PA W = F x d so W = PAd since V = Ad when a piston expands: W = PV Isobaric Process V = Vf - Vi so expansion of gas causes work done by system (+W) compression of gas caused by work done on system (-W) and this work is W = PV Work by the system Gas expands Isochoric Process Isovolumetric one in which volume doesn’t change since W = PV and there is no V process does no work on surrounding all heat added changes internal Internal energy energy increases U = Q No movement of piston Heat added PV Diagram Graph of the relationship between pressure and volume at various temperatures isotherms--lines (curves) with same temperature area under the curve (PV) gives you work done as conditions change for the various processes PV Diagram isothermic adiabatic isobaric isochoric volume 15-3 Human Metabolism and the First Law Metabolism--the many energy transforming processes that occur within an organism U = Q - W our bodies does work on environment and loses heat so to maintain our internal energy we must increase U by eating metabolic rate--rate at which U is transformed inside our body (kcal/h or watts) 15-4 The Second Law of Thermodynamics The Second Law of Thermodynamics it as many equivalent statements Clausius statement--heat flows naturally from a hot object to a cold object, but not spontaneously (by itself without input of some kind of work) from cold to hot the study of heat engines (any device that changes thermal energy into mechanical energy) will help develop a more general form 15-5 Heat Engines Any device that changes thermal energy into mechanical work the basic idea behind a heat engine is that mechanical energy can be obtained from thermal energy only when heat is allowed to flow from a high temperature to a cold temperature 15-5 Heat Engines Heat input (QH) at a High Temp TH high temperature (TH) is partly transformed QH into work (W) and partly exhausted as engine heat (QL) at a lower W temperature (TL) TH and TL are called the QL operating temperatures of the engine Low Temp TL 15-5 Heat Engines We will examine heat engines that run in a repeating cycle; continuously ****Note*** New Sign Convention: QH, QL, and W are always positive now Two types of practical engines: Steam engine Internal combustion engine 15-5 Heat Engines Steam engine Steps (OH): high pressure steam is produced by combustion or nuclear fission steam turns turbine or expands piston (work) low pressure steam is condensed and re-vaporized 15-5 Heat Engines Internal combustion engine steps (four-stroke engine)(OH): intake stroke--fuel mixture enters cylinder compression stroke--mixture is compressed by piston power stroke--fuel mixture is ignited and expanding gases force piston down (work) exhaust stroke-exhaust gas pushed from cylinder 15-5 Heat Engines Why is T needed to drive a heat engine? Efficiency of heat engine e = W/QH QH = W + QL (energy conserved) so W = QH - QL e = W/QH = (QH - QL)/QH = 1- QL/QH 15-5 Heat Engines Carnot Engine ideal engine each process of heat addition and exhaust, expansion or compression would be reversible (each step done slow enough to achieve equilibrium before next step occurs) real engines--process acts quickly, irreversible (due to friction and turbulence) 15-5 Heat Engines Carnot cycle four step cycle (OH) gas is expanded isothermally with addition of heat (T constant) gas expands adiabatically (no Q added but T) gas compressed isothermally gas expands adiabatically until its at original state 15-5 Heat Engines Carnot efficiency (ideal) eideal = (TH - TL)/TH = 1- TL/TH theoretical limit to efficiency 60-80% efficiency is excellent 15-5 Heat Engines Second Law of Thermodynamics Kelvin-Planck statement no device is possible whose sole effect is to transform a given amount of heat completely into work only possible if TL = 0K Third Law of Thermodynamics absolute zero is unattainable 15-6 Refrigerators, Air Conditioners, and Heat Pumps Operation of these devices is the reverse of a heat engine engine by doing work heat is taken from a low temperature region to a high temperature region High Temp TH QH W QL Low Temp TL 15-6 Refrigerators, Air Conditioners, and Heat Pumps Refrigerators and Air Conditioners process inside (low T) expanding gas absorbs heat; liquidgas condenser/pump; pumps warm gas outside and compresses it so that it loses heat; gas liquid 15-6 Refrigerators, Air Conditioners, and Heat Pumps Refrigerators and Air Conditioners coefficient of performance (CP) CP = QL/W = QL/(QH - QL) CPideal = TL/(TH- TL) 15-6 Refrigerators, Air Conditioners, and Heat Pumps Heat Pump device that heats a house by taking heat from QL (low T) outside house and delivering heat QH (high T) to warm inside of house works like refrigerator and A/C, can be reversed to cool in summer CP = QH/W 15-7 Entropy and the Second Law of Thermodynamics Entropy (S) is a state function of a system we deal with a change in entropy (S) during processes according to Clausius, the change in entropy of a system when an amount of heat (Q) is added to it by a reversible process at a constant temperature (K) is: S =Q/T 15-7 Entropy and the Second Law of Thermodynamics Entropy (S) if the temperature change is not too great an average value for temperature can be used, if not that’s what they made calculus for Second Law of Thermodynamics (again) the entropy of an isolated system never decreases. It can only stay the same or increase 15-7 Entropy and the Second Law of Thermodynamics Entropy (S) Second Law of Thermodynamics (and again) the entropy of an isolated system never decreases. It can only stay the same or increase for real processes; S>0 if system is not isolated: S = Ss + Senv0 15-7 Entropy and the Second Law of Thermodynamics Entropy (S) Second Law of Thermodynamics (and again) the total entropy of any system plus that of its environment increases as a result of any natural process only in ideal processes is S = 0 entropy is not conserved it is always increasing 15-8 Order to Disorder Entropy--a measure of the disorder of a system Second Law of Thermodynamics (once again) Natural processes tend to move toward a state of greater disorder Examples: salt/pepper, broken cup, mixing hot/cold 15-8 Order to Disorder An increase in entropy corresponds to an increase in disorder (randomness) Information theory built on the idea that the more orderly an arrangement the more information is needed to specific it or classify it hot/cold liquidscool liquid 15-9 Unavailability of Energy; Heat Death In the process of heat conduction from a hot body to a cold one entropy increases and order goes to disorder the separation of hot/cold objects can serve as high/low temperature regions for a heat engine and be used to obtain useful work when the two objects reach the same temperature no work can be obtained 15-9 Unavailability of Energy; Heat Death Second Law of Thermodynamics in any natural process, some energy becomes unavailable to do useful work in any process energy is never lost but it becomes less useful to do work energy is degraded as it goes from more ordered forms (mechanical) to less ordered forms (internal to thermal) 15-9 Unavailability of Energy; Heat Death A natural outcome is that as time goes on, the universe will approach a state of maximum disorder matter will become a uniform mixture at one temperature, no work can then be done all energy would be degraded to thermal energy and all change would cease (Heat Death) this is based on the assumption that the universe is finite 15-10 Evolution and Growth; “Time’s Arrow” Evolution of organisms and entropy Why has entropy called time’s arrow? 15.12 Energy Resources: Thermal Pollution Know this: greenhouse effect thermal pollution Processes for electricity generation Fossil-fuel steam plants Nuclear energy Geothermal energy Hydroelectric power plants Tidal energy Wind power Solar energy (solar cell/photovoltaic cell)