Chap-12A_Basic-Thermo-and-Laws
... – Property: characteristic of system such as temperature, pressure,… – State: condition of a system as described by its properties. • Any property change RESULTS in state changes – Process: a change in state (one or more properties change). • It is related to path followed – Extensive and intensive ...
... – Property: characteristic of system such as temperature, pressure,… – State: condition of a system as described by its properties. • Any property change RESULTS in state changes – Process: a change in state (one or more properties change). • It is related to path followed – Extensive and intensive ...
NOTES ON THERMODYNAMIC FORMALISM
... Interactions between systems are said to be reversible if they proceed infinitesimally slowly as a result of infinitesimal differences in temperature or pressure. Thus an infinitesimal change in the temperature of pressure of the systems would cause the interactions to occur in the opposite directio ...
... Interactions between systems are said to be reversible if they proceed infinitesimally slowly as a result of infinitesimal differences in temperature or pressure. Thus an infinitesimal change in the temperature of pressure of the systems would cause the interactions to occur in the opposite directio ...
Structure of Thrmodynamics
... Assume that the theorem is incorrect then there should be at least one case where a the stable state changes while the only external effect is a rise in a level of a weight. It possible to lower the weight and impart a velocity to the system. In this case the net external effect is zero while the st ...
... Assume that the theorem is incorrect then there should be at least one case where a the stable state changes while the only external effect is a rise in a level of a weight. It possible to lower the weight and impart a velocity to the system. In this case the net external effect is zero while the st ...
First Law of Thermodynamics - Derry Area School District
... • The entropy of an isolated system increases for every natural process. However, if a system is not isolated it may undergo a decrease in entropy. example for an isolated system: freezing ice. Water at room temperature will not spontaneously make ice. To freeze ice, we need to remove heat. This cre ...
... • The entropy of an isolated system increases for every natural process. However, if a system is not isolated it may undergo a decrease in entropy. example for an isolated system: freezing ice. Water at room temperature will not spontaneously make ice. To freeze ice, we need to remove heat. This cre ...
Chemistry 205 - Introductory General Chemistry
... the grade in this class is based on homework, so it is vital that you make your best attempt on every problem. If you are not sure about a problem please come ask, I am always willing to help. A homework assignment will be given every week except exam weeks. These are to be turned in at the end of t ...
... the grade in this class is based on homework, so it is vital that you make your best attempt on every problem. If you are not sure about a problem please come ask, I am always willing to help. A homework assignment will be given every week except exam weeks. These are to be turned in at the end of t ...
Lecture August 28
... Equilibrium state ☛ properties of system are uniform throughout and don’t change in time unless system is acted upon by external influences Non-Equilibrium state ☛ characterizes a system in which gradients exist and whose properties vary with time State variables ☛ properties that describes equilibr ...
... Equilibrium state ☛ properties of system are uniform throughout and don’t change in time unless system is acted upon by external influences Non-Equilibrium state ☛ characterizes a system in which gradients exist and whose properties vary with time State variables ☛ properties that describes equilibr ...
The Boltzmann distribution law and statistical thermodynamics
... Statistical mechanics is the theoretical apparatus with which one studies the properties of macroscopic systems – systems made up of many atoms or molecules – and relates those properties to the system’s microscopic constitution. One branch of the subject, called statistical thermodynamics, is devot ...
... Statistical mechanics is the theoretical apparatus with which one studies the properties of macroscopic systems – systems made up of many atoms or molecules – and relates those properties to the system’s microscopic constitution. One branch of the subject, called statistical thermodynamics, is devot ...
Thermo applications
... As will be shown in the next section, the energy required for any steady-state flow process is essentially the difference in enthalpy between the products and reactants, plus the amount of energy lost to the surroundings. The change in enthalpy over the process is easily calculated if the enthalpie ...
... As will be shown in the next section, the energy required for any steady-state flow process is essentially the difference in enthalpy between the products and reactants, plus the amount of energy lost to the surroundings. The change in enthalpy over the process is easily calculated if the enthalpie ...
Different levels of reversibility
... The internal energy of a system depends on the state of the system. The first law used the change in internal energy as one term. For any two given states, however, the first law does not care which state is the initial state and which state is the final state. It does not even care it if is possibl ...
... The internal energy of a system depends on the state of the system. The first law used the change in internal energy as one term. For any two given states, however, the first law does not care which state is the initial state and which state is the final state. It does not even care it if is possibl ...
lecture1424085736
... 4. Electric current with zero resistance. IRREVERSIBLE PROCESS An irreversible process is one that is carried out in such a way that the system and surrounding can not be exactly restored to their respective initial state at the end of the reverse process, that a net change occurs in the Universe. N ...
... 4. Electric current with zero resistance. IRREVERSIBLE PROCESS An irreversible process is one that is carried out in such a way that the system and surrounding can not be exactly restored to their respective initial state at the end of the reverse process, that a net change occurs in the Universe. N ...
Lecture12
... • Example 12.5 : Work and engine cylinder In a car engine operating at 1.80x103 rev/min, the expansion of hot, high-pressure gas against a piston occurs in about 10 ms. because energy transfer by heat typically takes a time on the order of minutes or hours, it is safe to assume that little energy le ...
... • Example 12.5 : Work and engine cylinder In a car engine operating at 1.80x103 rev/min, the expansion of hot, high-pressure gas against a piston occurs in about 10 ms. because energy transfer by heat typically takes a time on the order of minutes or hours, it is safe to assume that little energy le ...
2.2 Thermoelasticity
... In this section, thermoelasticity is considered. By definition, the constitutive relations for such a material depend only on the set of field variables F, , Grad . This general case will be considered toward the end of this section. Simpler cases, isothermal F , smallstrain ε, one-dimension ...
... In this section, thermoelasticity is considered. By definition, the constitutive relations for such a material depend only on the set of field variables F, , Grad . This general case will be considered toward the end of this section. Simpler cases, isothermal F , smallstrain ε, one-dimension ...
Notes on the second law of thermodynamics
... The internal energy of a system depends on the state of the system. The first law used the changed in internal energy as one term. For any two given states, however, the first law does not care which state is the initial state and which state is the final state. It does not even care it if is possib ...
... The internal energy of a system depends on the state of the system. The first law used the changed in internal energy as one term. For any two given states, however, the first law does not care which state is the initial state and which state is the final state. It does not even care it if is possib ...
Chapter Two The Thermodynamic Laws
... The first theory on the conversion of heat into mechanical work is due to Nicolas Léonard Sadi Carnot in 1824. Rudolf Clausius was the first to formulate the second law in 1850. Established in the 19th century, the Kelvin-Planck statement of the Second Law says, "It is impossible for any device that ...
... The first theory on the conversion of heat into mechanical work is due to Nicolas Léonard Sadi Carnot in 1824. Rudolf Clausius was the first to formulate the second law in 1850. Established in the 19th century, the Kelvin-Planck statement of the Second Law says, "It is impossible for any device that ...
Thermodynamics of dilute gases
... independent of their physical properties and the special kind of contact. Just this property is used to bring a substance to a given temperature, namely, by surrounding it with a heat bath. Then, by definition, substance and heat bath have the same temperature. To measure the temperature one can emp ...
... independent of their physical properties and the special kind of contact. Just this property is used to bring a substance to a given temperature, namely, by surrounding it with a heat bath. Then, by definition, substance and heat bath have the same temperature. To measure the temperature one can emp ...
Chapter 20
... Thermodynamic process ― a process in which the state variables of a thermodynamic system are changed. (Examples: pressure changed, or temperature changed, or both changed but with some other condition satisfied, etc.) Reversible processes ― thermodynamic processes which can also be run backward in t ...
... Thermodynamic process ― a process in which the state variables of a thermodynamic system are changed. (Examples: pressure changed, or temperature changed, or both changed but with some other condition satisfied, etc.) Reversible processes ― thermodynamic processes which can also be run backward in t ...
Lecture 9
... Examples: pressure, volume, internal energy, temperature. Also, entropy. Each variable on it’s own is not enough to determine the state of the system. (Many systems in different states might have the same volume.) Once the current state is known, we do know all of these variables’ values. How the sy ...
... Examples: pressure, volume, internal energy, temperature. Also, entropy. Each variable on it’s own is not enough to determine the state of the system. (Many systems in different states might have the same volume.) Once the current state is known, we do know all of these variables’ values. How the sy ...
8.5 CONVECTION By convection we mean a motion of material due
... By convection we mean a motion of material due to buoyancy forces resulting from temperature differences. cold hot material is less dense than cold material. It rises hot The theory of convection is too complicated to get into here, but we may still understand the basic ideas. The first one we need ...
... By convection we mean a motion of material due to buoyancy forces resulting from temperature differences. cold hot material is less dense than cold material. It rises hot The theory of convection is too complicated to get into here, but we may still understand the basic ideas. The first one we need ...
8.5 CONVECTION By convection we mean a motion of material due
... By convection we mean a motion of material due to buoyancy forces resulting from temperature differences. cold hot material is less dense than cold material. It rises hot The theory of convection is too complicated to get into here, but we may still understand the basic ideas. The first one we need ...
... By convection we mean a motion of material due to buoyancy forces resulting from temperature differences. cold hot material is less dense than cold material. It rises hot The theory of convection is too complicated to get into here, but we may still understand the basic ideas. The first one we need ...
Fundamentals of Energy Conversion
... In contrast, properties that may vary from point to point and that do not change with the mass of the system are called intensive properties. Temperature and pressure are well-known examples. For instance, thermometers at different locations in a system may indicate differing temperatures. But if a ...
... In contrast, properties that may vary from point to point and that do not change with the mass of the system are called intensive properties. Temperature and pressure are well-known examples. For instance, thermometers at different locations in a system may indicate differing temperatures. But if a ...
chapter 3 thermodynamics of dilute gases
... substance contained in the cylinder. The equation of state is a functional relationship between the internal energy per unit mass, specific volume and pressure, P ( e, v ) . For a general substance an accurate equation of state is not a particularly easy relationship to come by and so most applicati ...
... substance contained in the cylinder. The equation of state is a functional relationship between the internal energy per unit mass, specific volume and pressure, P ( e, v ) . For a general substance an accurate equation of state is not a particularly easy relationship to come by and so most applicati ...
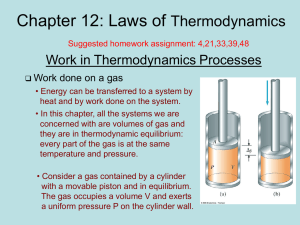
chapter12
... Grades of Energy, cont. • If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B • When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy • Degradation of energy is the conversi ...
... Grades of Energy, cont. • If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B • When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy • Degradation of energy is the conversi ...
Chapter 12 Notes
... Grades of Energy, cont. • If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B • When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy • Degradation of energy is the conversi ...
... Grades of Energy, cont. • If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B • When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy • Degradation of energy is the conversi ...
Provedení, principy činnosti a základy výpočtu pro výměníky tepla
... Be careful at interpretation: First law of thermodynamic was presented in the form corresponding only to reversible changes (therefore for infinitely slow changes, without viscous friction, at uniform temperature) ...
... Be careful at interpretation: First law of thermodynamic was presented in the form corresponding only to reversible changes (therefore for infinitely slow changes, without viscous friction, at uniform temperature) ...