Solutions Exercises Lecture 4
... the entropy of the subsystems A and B changes. Just as the enthalpy the entropy is a state function. Thus the change of the entropy of the complete system can be computed from the entropy changes of the subsystems: ∆Ssystem = ∆SA + ∆SB. In general the computation of the entropy change is based on a ...
... the entropy of the subsystems A and B changes. Just as the enthalpy the entropy is a state function. Thus the change of the entropy of the complete system can be computed from the entropy changes of the subsystems: ∆Ssystem = ∆SA + ∆SB. In general the computation of the entropy change is based on a ...
Course Solution Set 18-24
... using the facts that 1 ml. of water has a mass of one gram and that 1 K = 1 ºC. absolute. Thus, the final temperature of the water in the blender will be about 12.5 ºC. (this answer ! is approximate to the degree that our estimate of the amount of heating is approximate). b) For an object made of a ...
... using the facts that 1 ml. of water has a mass of one gram and that 1 K = 1 ºC. absolute. Thus, the final temperature of the water in the blender will be about 12.5 ºC. (this answer ! is approximate to the degree that our estimate of the amount of heating is approximate). b) For an object made of a ...
The Second Law of Thermodynamics
... air by absorbing heat from the floor. Such a process will not violate the first law. If the mass of the ball is m, and the height above the floor to which it rises is h, we have energy extracted from the floor ¼ mgh where g is acceleration due to gravity. The thermal energy of the floor is random molecul ...
... air by absorbing heat from the floor. Such a process will not violate the first law. If the mass of the ball is m, and the height above the floor to which it rises is h, we have energy extracted from the floor ¼ mgh where g is acceleration due to gravity. The thermal energy of the floor is random molecul ...
documentstyle[12pt]{article}
... The study of thermodynamics is concerned with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. One of the most fundamental laws of nature is the conservation of energy principle. It simply states that during an energy interaction, e ...
... The study of thermodynamics is concerned with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. One of the most fundamental laws of nature is the conservation of energy principle. It simply states that during an energy interaction, e ...
Inexistence of equilibrium states at absolute negative temperatures
... temperature, thus proving that the former are hotter than the latter. In the Appendix, we provide the theorems needed to corroborate these statements. But before arguing that states at presumably negative temperature are not stable equilibrium states, we would like to point out that a world with neg ...
... temperature, thus proving that the former are hotter than the latter. In the Appendix, we provide the theorems needed to corroborate these statements. But before arguing that states at presumably negative temperature are not stable equilibrium states, we would like to point out that a world with neg ...
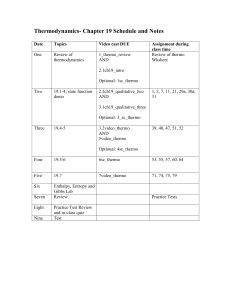
Schedule and sample problems
... page. Write neat and large and use lots of white space on this and other pages provided by you. ...
... page. Write neat and large and use lots of white space on this and other pages provided by you. ...
Chapter 1
... thermodynamics is equivalent to law of conservation of energy. It deals with the transformation of heat energy into work and vice versa. oWhen a small amount of work (dw) is supplied to a closed system undergoing a cycle, the work supplied will be equal to the heat transfer or heat produced (dQ) in ...
... thermodynamics is equivalent to law of conservation of energy. It deals with the transformation of heat energy into work and vice versa. oWhen a small amount of work (dw) is supplied to a closed system undergoing a cycle, the work supplied will be equal to the heat transfer or heat produced (dQ) in ...
Chapter 1 - All Made Easy
... thermodynamics is equivalent to law of conservation of energy. It deals with the transformation of heat energy into work and vice versa. oWhen a small amount of work (dw) is supplied to a closed system undergoing a cycle, the work supplied will be equal to the heat transfer or heat produced (dQ) in ...
... thermodynamics is equivalent to law of conservation of energy. It deals with the transformation of heat energy into work and vice versa. oWhen a small amount of work (dw) is supplied to a closed system undergoing a cycle, the work supplied will be equal to the heat transfer or heat produced (dQ) in ...
Chapter 12: Engineering Thermodynamics
... some way by which both the system and its surroundings can be exactly restored to their respective initial states. A process is irreversible if both the system and surroundings cannot be restored to their initial states. There are many effects whose presence during a process renders it irreversible. ...
... some way by which both the system and its surroundings can be exactly restored to their respective initial states. A process is irreversible if both the system and surroundings cannot be restored to their initial states. There are many effects whose presence during a process renders it irreversible. ...
Cellular Thermodynamics
... measurable. The complementary discipline of statistical mechanics applies the laws of physics to individual molecules, atoms and photons and, by considering the statistical behaviour of large numbers, deduces the behaviour of macroscopic systems. It thus relates thermodynamics to Newtonian or quantu ...
... measurable. The complementary discipline of statistical mechanics applies the laws of physics to individual molecules, atoms and photons and, by considering the statistical behaviour of large numbers, deduces the behaviour of macroscopic systems. It thus relates thermodynamics to Newtonian or quantu ...
Review of classical thermodynamics
... in the system). But there are too many particles (NA = 6.022×1023 mol-1) and this approach in unpractical in most cases. 2. Classical (continuum) thermodynamics – to describe the material in terms of average quantities, or thermodynamic variables, such as temperature, internal energy, pressure, etc. ...
... in the system). But there are too many particles (NA = 6.022×1023 mol-1) and this approach in unpractical in most cases. 2. Classical (continuum) thermodynamics – to describe the material in terms of average quantities, or thermodynamic variables, such as temperature, internal energy, pressure, etc. ...
On the Foundations of Classical Thermodynamics, and the Tolman
... the state description are coarse-grained over by statistical averaging, in a way exemplified by the central limit theorem, and the quantities of thermodynamics are then explained as emerging from the statistical action of matter in aggregation [5] (p.455). The phenomenological approach however, whic ...
... the state description are coarse-grained over by statistical averaging, in a way exemplified by the central limit theorem, and the quantities of thermodynamics are then explained as emerging from the statistical action of matter in aggregation [5] (p.455). The phenomenological approach however, whic ...
Word document format
... The sign of q, heat, or work, w, indicates the direction of the flow of energy. The currently accepted sign convention is that if heat flows out the system to the surroundings, q is negative. If one were carrying out a reaction in a test tube, the test tube would feel warmer. If heat flows into the ...
... The sign of q, heat, or work, w, indicates the direction of the flow of energy. The currently accepted sign convention is that if heat flows out the system to the surroundings, q is negative. If one were carrying out a reaction in a test tube, the test tube would feel warmer. If heat flows into the ...
Тепломассообмен
... in constant motion, colliding and rebounding not unlike billiard balls. To describe matter the history of each molecule must be known. This requires knowing each molecule’s velocity and acceleration which is quite impossible except statistically. In engineering applications, however, we are interest ...
... in constant motion, colliding and rebounding not unlike billiard balls. To describe matter the history of each molecule must be known. This requires knowing each molecule’s velocity and acceleration which is quite impossible except statistically. In engineering applications, however, we are interest ...
slides - Biology Courses Server
... Another Kind of State Function w and q are not state functions. BUT, we can define the value of w (or q) for a specific process linking two states to be a change in a state function. We define the work for the reversible (infinitely slow) conversion of one state to the another, wrev , to be the cha ...
... Another Kind of State Function w and q are not state functions. BUT, we can define the value of w (or q) for a specific process linking two states to be a change in a state function. We define the work for the reversible (infinitely slow) conversion of one state to the another, wrev , to be the cha ...
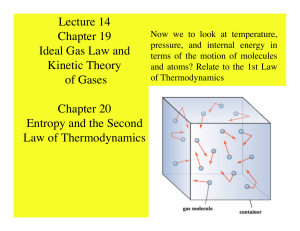
Lecture 14 Chapter 19 Ideal Gas Law and Kinetic Theory of Gases
... A system tends to move in the direction where entropy increases • For an irreversible process the entropy always increases. ΔS > 0 • For a reversible process it can be 0 or increase. ΔS ≥ 0 ...
... A system tends to move in the direction where entropy increases • For an irreversible process the entropy always increases. ΔS > 0 • For a reversible process it can be 0 or increase. ΔS ≥ 0 ...
ISAT 310: Energy Fundamentals
... Flow through such devices is best studied by selecting the region within the device as the control volume . Both mass and energy can cross the boundary of the control volume. There are no concrete rules for the selection of the control volume but proper choice makes the analysis much easier . The bo ...
... Flow through such devices is best studied by selecting the region within the device as the control volume . Both mass and energy can cross the boundary of the control volume. There are no concrete rules for the selection of the control volume but proper choice makes the analysis much easier . The bo ...
2. THERMODYNAMICS and ENSEMBLES (Part A) Introduction
... calculate parameters of macroscopic significance. This is the method of “kinetic theory”. It can yield results even for system not in equilibrium. This is the most difficult method to apply because it attempt to yield such detailed description. In the present chapter, we shall confine ourselves to t ...
... calculate parameters of macroscopic significance. This is the method of “kinetic theory”. It can yield results even for system not in equilibrium. This is the most difficult method to apply because it attempt to yield such detailed description. In the present chapter, we shall confine ourselves to t ...
Chapter 16 - Faculty Server Contact
... • Each one can be used to prove the other two • Each version gives different insights into the meaning and consequences of the second law ...
... • Each one can be used to prove the other two • Each version gives different insights into the meaning and consequences of the second law ...
Lecture 3: FIRST LAW OF THERMODYNAMICS
... Calculate the final temperature when cold ice is dropped into hot water. Proceed by assuming a series of processes in which the energy goes into some unspecified storage reservoir The water cools to 0◦ . The ice warms to 0◦ . The ice melts. Finally, the sum of these energies is put back into the sys ...
... Calculate the final temperature when cold ice is dropped into hot water. Proceed by assuming a series of processes in which the energy goes into some unspecified storage reservoir The water cools to 0◦ . The ice warms to 0◦ . The ice melts. Finally, the sum of these energies is put back into the sys ...
Thermochemistry, thermodynamics Thermochemistry
... measuring the heat of chemical reactions or physical changes as well as heat capacity. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. ...
... measuring the heat of chemical reactions or physical changes as well as heat capacity. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. ...
Document
... The study of thermodynamics is concerned with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. One of the most fundamental laws of nature is the conservation of energy principle. It simply states that during an energy interaction, e ...
... The study of thermodynamics is concerned with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. One of the most fundamental laws of nature is the conservation of energy principle. It simply states that during an energy interaction, e ...
Chemical Thermodynamics John Murrell Introduction
... A precise definition of temperature, and its measure by the kelvin scale, 0K = 273.15C (one cannot have a temperature below 0K), arises from the second law of thermodynamics, which we examine later. The kelvin scale also comes from the statistical theory developed by Boltzmann in which thermodynamic ...
... A precise definition of temperature, and its measure by the kelvin scale, 0K = 273.15C (one cannot have a temperature below 0K), arises from the second law of thermodynamics, which we examine later. The kelvin scale also comes from the statistical theory developed by Boltzmann in which thermodynamic ...
NATURE OF ENTROPY OF MIXING
... change of weight in one or other direction. In all the accomplished experiments when energy was introduced into a body (heating, deformation etc.) the weight decreased, while in reverse processes, viz., cooling, crystallization, it increased which corroborated the above hypothesis that weight change ...
... change of weight in one or other direction. In all the accomplished experiments when energy was introduced into a body (heating, deformation etc.) the weight decreased, while in reverse processes, viz., cooling, crystallization, it increased which corroborated the above hypothesis that weight change ...


![documentstyle[12pt]{article}](http://s1.studyres.com/store/data/010234315_1-392ad57a1bf5b2aaeca94206588a5307-300x300.png)