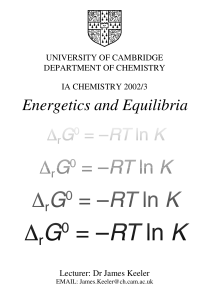
Intermediate Physical Chemistry (CHEM2503)
... Therefore, the internal energy U may be expressed as U = U(0) + E = U(0) - N (lnq/)V Where, U(0) is the internal energy of the system at T = 0. The above equation provides the energy as a function of various properties of the molecular system (for instance, temperature, volume), and may be used t ...
... Therefore, the internal energy U may be expressed as U = U(0) + E = U(0) - N (lnq/)V Where, U(0) is the internal energy of the system at T = 0. The above equation provides the energy as a function of various properties of the molecular system (for instance, temperature, volume), and may be used t ...
Energetics and Equilibria
... The Master Equations..............................................................................................................7 Maxwell's relations...................................................................................................................7 Appendix: Formal proofs ........ ...
... The Master Equations..............................................................................................................7 Maxwell's relations...................................................................................................................7 Appendix: Formal proofs ........ ...
Basic Thermodynamics - Alpha College of Engineering
... steam point, but else where they are related by T A=p+qTB+rTB2, where p,q and r are constants. When the thermometers are immersed in an oil bath, A shows a temperature of 51 0C, while B shows 500C. Determine the temperature TA, when TB is 250C. [04 Marks, June-2015] ...
... steam point, but else where they are related by T A=p+qTB+rTB2, where p,q and r are constants. When the thermometers are immersed in an oil bath, A shows a temperature of 51 0C, while B shows 500C. Determine the temperature TA, when TB is 250C. [04 Marks, June-2015] ...
Heat Engines, Entropy, and the Second Law of Thermodynamics
... existence of such a device would be in violation of the second law of thermodynamics, which in the form of the Clausius statement 3 states: It is impossible to construct a cyclical machine whose sole effect is to transfer energy continuously by heat from one object to another object at a higher temp ...
... existence of such a device would be in violation of the second law of thermodynamics, which in the form of the Clausius statement 3 states: It is impossible to construct a cyclical machine whose sole effect is to transfer energy continuously by heat from one object to another object at a higher temp ...
Thermodynamic Cycles Knowledge Check
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
Thermodynamic Cycles Knowledge Check
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
Thermodynamic Cycles Knowledge Check
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
... We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapor such as stea ...
Chapter 19 Thermodynamics - Farmingdale State College
... at point C until it reaches the point D. The pressure of the gas is then increased from pD to pA while the volume of the gas in the cylinder is kept constant. This is shown as the path DA in the p-V diagram. Now let us assume that the points A, C, and D are the same points that were considered in fi ...
... at point C until it reaches the point D. The pressure of the gas is then increased from pD to pA while the volume of the gas in the cylinder is kept constant. This is shown as the path DA in the p-V diagram. Now let us assume that the points A, C, and D are the same points that were considered in fi ...
Thermodynamics Theory + Questions.0001
... • All statess of the syste em passes th hrough are equillibrium state es. • If we rem move the weig ghts slowly one o by Fig. A quasi q – static process one the pressure of the gass will displace the piston gradually. It is quasista atic. • On the other hand if we remove all a the weights at once th ...
... • All statess of the syste em passes th hrough are equillibrium state es. • If we rem move the weig ghts slowly one o by Fig. A quasi q – static process one the pressure of the gass will displace the piston gradually. It is quasista atic. • On the other hand if we remove all a the weights at once th ...
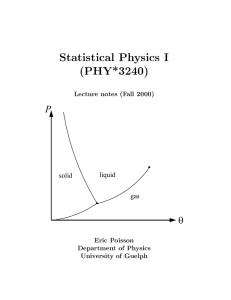
book - University of Guelph Physics
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
Prediction of the Steady Rate of Flame Spread Over
... spread process [I-41, provides a most valuable information on the overall effect of flame propagation esspecially in the sense of practical application to fire safety. Furthermore, the flame spread velocity is the single parameter that can be measured directly, quite accurately and rather easily in ...
... spread process [I-41, provides a most valuable information on the overall effect of flame propagation esspecially in the sense of practical application to fire safety. Furthermore, the flame spread velocity is the single parameter that can be measured directly, quite accurately and rather easily in ...
6. Macroscopic equilibrium states and state variables (Hiroshi
... temperature scale,” which was later found to coincide with the absolute scale. Extending the concept of equilibrium state to include mechanical equilibrium In the above, we first defined the notion of equilibrium state for “isolated” systems, which cannot exchange energy with another system and whos ...
... temperature scale,” which was later found to coincide with the absolute scale. Extending the concept of equilibrium state to include mechanical equilibrium In the above, we first defined the notion of equilibrium state for “isolated” systems, which cannot exchange energy with another system and whos ...
12A The Laws of Thermodynamics (Notes Phy
... Another example: when a tornado hits a building, there is major damage. You never see a tornado approach a pile of rubble and leave a building behind when it passes. Thermal equilibrium is a similar process – the uniform final state has more disorder than the separate temperatures in the initial sta ...
... Another example: when a tornado hits a building, there is major damage. You never see a tornado approach a pile of rubble and leave a building behind when it passes. Thermal equilibrium is a similar process – the uniform final state has more disorder than the separate temperatures in the initial sta ...
S15--AP Phys Q4--Heat-Thermo Ch13_14_15
... Identify the choice that best completes the statement or answers the question. 1. Which of the following is a thermodynamic process in which a system returns to the same conditions under which it started? a. a cyclic process b. an isothermal process c. an isovolumetric process d. an adiabatic proces ...
... Identify the choice that best completes the statement or answers the question. 1. Which of the following is a thermodynamic process in which a system returns to the same conditions under which it started? a. a cyclic process b. an isothermal process c. an isovolumetric process d. an adiabatic proces ...
thermodynamics type 1
... of quantities of heat & work. It may be defined as the branch of science which deals with energy changes associated with various physical & chemical processes. The entire formulation of thermodynamics is based on a few (Three) fundamental laws which have been established on the basis of human experi ...
... of quantities of heat & work. It may be defined as the branch of science which deals with energy changes associated with various physical & chemical processes. The entire formulation of thermodynamics is based on a few (Three) fundamental laws which have been established on the basis of human experi ...
A survey of statistical mechanics as it pertains to molecular simulation
... energy Ei. Note that the weighting accorded to a microstate depends only on its energy; states of equal energy have the same weight. The normalization constant Q is very important, and will be discussed in more detail below. Note also that the quantity E/T, which appears in the exponent, in thermody ...
... energy Ei. Note that the weighting accorded to a microstate depends only on its energy; states of equal energy have the same weight. The normalization constant Q is very important, and will be discussed in more detail below. Note also that the quantity E/T, which appears in the exponent, in thermody ...
Document
... performed on the system, for example. In a refrigerator, heat flows from cold to hot, but only when forced by an external agent, a compressor. Kelvin statement The Kelvin statement expresses as follows: No process is possible in which the sole result is the absorption of heat from a reservoir and it ...
... performed on the system, for example. In a refrigerator, heat flows from cold to hot, but only when forced by an external agent, a compressor. Kelvin statement The Kelvin statement expresses as follows: No process is possible in which the sole result is the absorption of heat from a reservoir and it ...
Outline Introduction Introduction Gibbs Free Energy
... A further increase in pressure causes little further reduction in volume as liquid is generally not compressible to a large extent. When the P-V relationship is examined at different T, one may obtain the results shown in the diagram below: ...
... A further increase in pressure causes little further reduction in volume as liquid is generally not compressible to a large extent. When the P-V relationship is examined at different T, one may obtain the results shown in the diagram below: ...
Thermodynamics with Chemical Engineering Applications
... First introduction of the Helmholtz and Gibbs free energy functions. First and Second Laws combined in four versions 8.3 Dependence of S, U, H, A, and G on T, p, and V. Maxwell’s relations 8.3.1 Entropy vs. p–V–T 8.3.2 Internal energy vs. p–V –T 8.3.3 Enthalpy vs. p–V –T 8.3.4 Helmholtz free energy ...
... First introduction of the Helmholtz and Gibbs free energy functions. First and Second Laws combined in four versions 8.3 Dependence of S, U, H, A, and G on T, p, and V. Maxwell’s relations 8.3.1 Entropy vs. p–V–T 8.3.2 Internal energy vs. p–V –T 8.3.3 Enthalpy vs. p–V –T 8.3.4 Helmholtz free energy ...
Constructor Theory of Thermodynamics
... thermodynamics and constructor-theoretic information theory. Central to this paper is the difference between a task being possible and a process being permitted by dynamical laws. The latter means that the process occurs spontaneously (i.e., when the physical system has no interactions with the surr ...
... thermodynamics and constructor-theoretic information theory. Central to this paper is the difference between a task being possible and a process being permitted by dynamical laws. The latter means that the process occurs spontaneously (i.e., when the physical system has no interactions with the surr ...
ee11042602mpt3.mov 110426ph423main3.mov Example of the
... a gas at fixed volume, and would naturally be done with a bomb calorimeter, much as the students describe. He would have a heating element, and measure how much power it outputs in a given time interval (to measure the energy put into the system), and then see how much the temperature of the gas cha ...
... a gas at fixed volume, and would naturally be done with a bomb calorimeter, much as the students describe. He would have a heating element, and measure how much power it outputs in a given time interval (to measure the energy put into the system), and then see how much the temperature of the gas cha ...
Heat Engines, Entropy, and the Second Law of Thermodynamics P
... possible. To understand its nature, we must first examine the meaning of reversible and irreversible processes. In a reversible process, the system undergoing the process can be returned to its initial conditions along the same path shown on a PV diagram, and every point along this path is an equili ...
... possible. To understand its nature, we must first examine the meaning of reversible and irreversible processes. In a reversible process, the system undergoing the process can be returned to its initial conditions along the same path shown on a PV diagram, and every point along this path is an equili ...
Document
... where q i exp(-i) is the molecular partition function. The second equality is satisfied because the molecules are independent of each other. The above equation applies only to molecules that are distinguishable, for instance, localized molecules. However, if the molecules are identical and free ...
... where q i exp(-i) is the molecular partition function. The second equality is satisfied because the molecules are independent of each other. The above equation applies only to molecules that are distinguishable, for instance, localized molecules. However, if the molecules are identical and free ...
Chapter 2
... As we discussed in Chapter 1 the macrostate of a system refers to bulk properties such as temperature and pressure. Only a few quantities are needed to specify the macrostate of a system in equilibrium. For example, if you drop an ice cube into a cup of coffee, the temperature immediately afterward ...
... As we discussed in Chapter 1 the macrostate of a system refers to bulk properties such as temperature and pressure. Only a few quantities are needed to specify the macrostate of a system in equilibrium. For example, if you drop an ice cube into a cup of coffee, the temperature immediately afterward ...
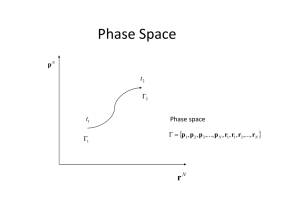
Phase Space Phase Space
... initial conditions is not considered; thus, p(t), q(t) are not sought. Instead, the motion of a whole set of phase points, representing the collection of possible states of the given system. Such a set of phase points is called a phase space ensemble. If each point in the phase space is considered a ...
... initial conditions is not considered; thus, p(t), q(t) are not sought. Instead, the motion of a whole set of phase points, representing the collection of possible states of the given system. Such a set of phase points is called a phase space ensemble. If each point in the phase space is considered a ...