* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download New Microsoft Office Word Document
State of matter wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
R-value (insulation) wikipedia , lookup
Black-body radiation wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Equipartition theorem wikipedia , lookup
Calorimetry wikipedia , lookup
Heat capacity wikipedia , lookup
Thermoregulation wikipedia , lookup
Thermal radiation wikipedia , lookup
Conservation of energy wikipedia , lookup
Equation of state wikipedia , lookup
Heat transfer wikipedia , lookup
Heat equation wikipedia , lookup
Temperature wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Thermal conduction wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Gibbs free energy wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Adiabatic process wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Thermodynamics
System:- Observed part of universe
State of the system:- the existence of system with its respective
microscopic and macroscopic properties
Surrounding:- Part of universe apart from system
Universe:- System along with all the surroundings
Boundary:- Walls that separate System from Surroundings
Equilibrium:- A state of dynamics wherein all observable properties are
constant
Thermodynamic Equilibrium:- A system in which all macroscopic
properties do not undergo any change with time
Thermal Equilibrium:- If there is no heat exchange from one portion of
the system to the another portion of the system, the system is said to
be in Thermal Equilibrium
Mechanical Equilibrium:- If no work is done by one part of the system
on other part of the system, it is termed as Mechanical Equilibrium
Chemical Equilibrium:- If the rate of forward reaction equals the rate of
backward reaction in the reversible conversion of some reactants to
products in a closed container it is called Chemical Equilibrium
Types of System:-
System
Open System
Can exchange both
mass and heat with
surrounding
Example:- a rection
proceeding in a lid less
container
Closed System
Can only exchange heat
with the surrounding
Example:- a reaction
proceeding in a
container with a lid
Isolated System
Can exchange neither
mass nor heat with the
surrounding
Example:- a reaaction
proceeding in a closed
container with insulated
walls, AThermos flask
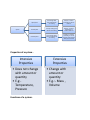
Properties of a system:-
Intensive
Properties
• Does not change
with amount or
quantity
• E.g:Temperature,
Pressure
Functions of a system:-
Extensive
Properties
• Change with
amount or
quantity
• E.g.:- Mass ,
Volume
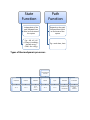
State
Function
Path
Function
Independent of the
path followed from
initial to final state of
the system
Depends on the path
followed from initial
to final state of the
system
E.g.:- ∆H, ∆U, ∆G
(Change in enthalpy,
internal energy,
Gibb's free energy
E.g:- work done, heat
Types of thermodynamic processes:-
Thermodynami
c Process
Isothermal
Isochoric
Adiabatic
Isobaric
Cyclic
Reversible
Irreversible
∆T= 0
∆V= 0
∆q= 0
TVγ-1 is
constant
∆P= 0
∆U= 0
Equilibrium can
be achieved
Equilibrium can
not be
achieved
Laws of thermodynamics (statements):-
ZEROTH LAW
If two systems are in equilibrium with a
same system externally then they are in
equilibrium with each other too.
FIRST LAW
Energy can neither be created nor be
destroyed, it can only be transformed
into varius forms.
SECOND LAW
A reversible chemical reaction can only be
reversed by introduction of an external
agency
THIRD LAW
The entropy of a crystalline solid at
absolute temperature(0K) is zero.
Laws of thermodynamics (mathematical expression)
Zeroth Law
• A↔B↔C
First Law
• ∆U=∆q+∆W
Second Law
• at 0K, ∆S=0
Third Law
• ∆S=2.303∫CPlog
dT
Internal Energy (U):- Sum total of all the energies of all molecules in a
system.
Internal energy cannot be determined rather the change in internal
energy (∆U) can be determined.
∆U is negative for exothermic reaction
∆U is positive for endothermic reaction
Internal energy depends on pressure, temperature, volume and
quantity
Work (W):- A form of energy. It can be defined as the product of
volume and difference between pressure of system and
surroundingoccurs in a gaseous matter.
It is a path function
Expression for various Thermodynamic Work :Irreversible, Isothermal work done and at constant pressure
WPV=-Pext.∆V
Reversible, Isothermal work done
Wrev=-2.303 nRTlog(V2/V1)
Reversible adiabatic work done:Wrev= Nr(T2-T1)/γ-1
Irreversible adiabatic work done
Wirrev=-Pext.R{(P1T2-P2T1)/P1P2}
SPECIAL POINT:Reversible work done is always greater than Irreversible work done
Work done and heat are seen only at the boundary of system and
surrounding at the time of change of state.
Heat Capacity (q):- Amount of heat needed to increase the
temperature of the system by 1̊C
Molar heat capacity (q/n):- Heat capacity for one mole of matter
Specific heat capacity (C or q/m):- Heat capacity for one gram of matter
Molar heat capacity at constant pressure= CP = 5/2R
Molar heat capacity at constant volume= CV = 3/2R
CP – CV = R
Poisson’s Ratio (γ) = CP/CV
Atomicity of the gas
Monoatomic
Diatomic
Triatomic
Value of γ
1.66
1.40
1.33
Enthalpy (H):- sum of internal energy and stored energy of a system
It is a state function and an extensive property
H=U+PV
∆H=∆U+P∆V
∆H=U+∆nRT
∆H is positive for endothermic reactions
∆H is negative for exothermic reactions
Enthalpy depends on the state of the system, allotropic forms of
matter, composition of system, amount of reactants and temperature
too
Entropy (S):- Degree of randomness
S=qrev/T
∆S= n.CV.ln(T2/T1)+n.R.ln(V2/V1)
Gibb’s free energy (G):- It is defined as the difference of enthalpy and
product of temperature with entropy.
G=H-TS
∆G=∆H- T∆S
∆G = 0 at equilibrium
∆G = ∆G̊ + R.T.lnK
At eqm, ∆G= -2.303.R.T.logK
∆G̊ = -nFE̊cell (for electrochemical cells)
SIGN CONVENTIONS AND SPONTANIETY OF A REACTION
Serial no.
∆H
∆S
∆G=∆H-T∆S
1
2
Negative
Positive
Positive
Negative
Negative
Positive
3
Positive
Positive
4
Negative
Negative
Low T,
Positive
High T,
Negative
Low T,
Negative
High T,
Positive
SOME OTHER LAWS
Lavoisier Laplace Law
A → B (H= ∆H1)
B→A (H=-∆H2)
Hess’s Law
A→B (H=∆H1)
A → C (H=∆H2) → D (H=∆H3) → B (H=∆H4)
∆H1 = ∆H2 + ∆H3 + ∆H4
Trouton’s Law
Reaction
type
Spontaneous
Non
spontaneous
Non
spontaneous
Spontaneous
Spontaneous
Non
spontaneous
∆HVap/Tboiling = 88J/mol/K
Dulong Petit Law
C*M = 6.4 cal ̊C/mol
Kirchoff’s Equation
∆CP = (∆H2 - ∆H2)/(T2 – T1)
∆CV = (∆U2 - ∆U2)/(T2 – T1)
Clausius Clapeyron Equation
-2.303 log(P2/P1) = ∆HVap/R{(T2 – T1)/T1T2}
Joule Thomson Effect:Adiabatic expansion of a gas from high pressure to low pressure causes
cooling of the gas
Joule Thomson coefficient (µ) :- dT/dP
SPECIAL POINT:Ideal gas expansion in vacuum witness no Joule Thomson effect
When the temperature goes beyond Inversion Temperature, Joule
Thomson coefficient is zero.
Inversion Temperature (Ti) = 2a/Rb