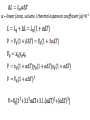* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture5
Conservation of energy wikipedia , lookup
Thermal conductivity wikipedia , lookup
R-value (insulation) wikipedia , lookup
Maximum entropy thermodynamics wikipedia , lookup
Heat capacity wikipedia , lookup
Calorimetry wikipedia , lookup
Thermal expansion wikipedia , lookup
Heat equation wikipedia , lookup
Black-body radiation wikipedia , lookup
Equipartition theorem wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat transfer wikipedia , lookup
State of matter wikipedia , lookup
Thermal radiation wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Temperature wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Thermal conduction wikipedia , lookup
Equation of state wikipedia , lookup
Heat transfer physics wikipedia , lookup
Van der Waals equation wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
Adiabatic process wikipedia , lookup
History of thermodynamics wikipedia , lookup
Lecture 5 Physics 2016/2017 Thermal physics Temperature – T (t) – Intrinsic property – independent on the amount of the material temperarure = average kinetic energy of molecules [T] = 1 K = 1oC T = t + 273.15 oC Measurement of temperature properties of materials varying with temperature • volume • electrical properties • pressure (gauges thermometers) • IR radiation…. • Temperature scales Kelvin – [T] = 1 K Celsius – [t] = 1 oC 0 oC = 273.15 K 100 oC = 373.15 K water freezing-boiling T1 K = (t1 + 273.15 ) oC T2 K = (t2 + 273.15 ) oC (T1 - T2) K = (t2 - t2 ) oC T=0K => p=0, V=0 impossible to reach • Thermal expansion of materials α – linear (area, volume ) thermal expanson coefficient [α]=K-1 • Density variation with temperature Density decreases with increasing temperature! • Anomaly of water density 0<t<3.98oC β<0, the density increases with t t=3.98oC β=0, density has maximum t> 3.98oC β>0, density decreases with t “normal” behaviour • Thermodynamics – branch of physics concerned with heat and temperature and their relation to energy and work Thermodynamic system is the content of a macroscopic volume in space, along with its walls and surroundings; it undergoes thermodynamic processes according to the principles of thermodynamics. Thermodynamic systems – • • • • open system – energy, matter exchange closed system – energy, matter exchange isolated system - energy, matter exchange adiabatic process – no heat or matter exchange, internal energy transfer to work State functions - their values do not depend on the path, done by the system to get to the given state (internal energy, entropy...) Process (path) functions – heat, work depend on the path intensive – independent on the number particles, T, p, Vm… extensive – dependent… V, n but Vm=V/n molar volume – intensive Vs=V/m specific volume - intensive State functions ΔX12= -ΔX21 Path function ΔXA= -ΔXB =- ΔXC ΔX= ΔX1 +ΔX2 +ΔX3 +ΔX4 Exact differential (total differential) example Ideal gas • the molecules are point-like particles • elastic collisions • no interactions between the particles, except the collisions Real gases behave similarly at high temperatures and low densities • molecules have non-zero volume • interactions • not allways elastic collisions • Brownian motion • Thermodynamic state – system fully identified with a suitable set of state variables (function) for examples: pressure p, volume V, temperature T, number of moles n. Gay-Lussac I. law, Charles’ law, Law of Volumes isobaric process State variables: V,p,T p = const. Pressure – temperature law, Gay-Lussac II.law isochoric process V= const. Boyle-Mariotte law isothermal process T=const. pV= const. Avogadro’s law Under the same conditions of temperature and pressure, equal volumes of different gases contain an equal number of molecules (regardless on their chemical nature and physical properties). NA= 6.02214129 × 1023 Vm=22,41 dm-3=22,41 l (T=273,15K,p= 101 325 Pa) Avogadro’s number or Avogadro constant. 𝑉 = 𝑛 𝑉𝑚 p=101 325 Pa, T= 273.15 K Vm = 22.41 L = 0.02241 m-3 NA=6.022x1023 Ideal gas law Gay-Lussac laws V= const . T p= const . T Boyle-Mariotte pV = const Avogadro V = const . n Ideal gas law pV=n R T R= 8.314 JK-1mol-1 k= R/NA = 1.3806 x 10-23 JK-1 pV=N kBT N=nNA universal gas constant Boltzmann constant kB–bridge from macroscopic to microscopic physics Dalton´s law – mixture of gases 𝑛= 𝑛𝑖 𝑖 𝑛𝑅𝑇 𝑝= = 𝑉 𝑖 𝑛𝑖 𝑅𝑇 = 𝑉 𝑝𝑖 𝑖 In a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. 𝑝𝑖 = 𝑛 𝑖 𝑅𝑇 𝑉 Each gas exerts the same pressure they would exert if they were in the container alone. Real gases Real gases – particles not point mass, real volume – not negligible interactions, attraction-repulsion, non-elastic collisions. van der Waals equation 𝑛2 𝑎 𝑝+ 2 𝑉 𝑛2𝑎 𝑉2 𝑉 − 𝑛𝑏 = 𝑛𝑅𝑇 - correction for non-elastic interactions, attraction- repulsion 𝑛𝑏 – correction for the real volume of particles a,b – material constants, determined experimentaly • Heat and temperature Q [Q] = 1 J (energy) transfer of heat – change of temperatures c – heat capacity [c] = J K-1 cm – molar heat capacity [cm] = J mol-1K-1 cs – specific heat capacity [cs] = J kg-1K-1 • Work of ideal gases (W, A) [W] = 1J W = -Fex Δx = -p S Δx = -p ΔV Sign convention – from the point of view of the system receives releases Q: + W=-∫pdV + ΔV<0 compression ΔV>0 expansion Zeroth law of thermodynamics If two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other. Thermodynamic equilibrium (steady) state equilibrium – stable, unstable, indifferent Thermodynamic equilibrium, condition or state of a thermodynamic system, the properties of which do not change with time and that can be changed to another condition only at the expense of effects on other systems. entropy (at given energy)- maximum, Gibbs free energy (at given p, T) - minimum Reversible process – is a process that can be "reversed" by means of infinitesimal changes in some property of the system. A reversible process does not increase entropy of the system and surroundings. During a reversible process, the system is in thermodynamic equilibrium with its surroundings throughout the entire process. Real processes are not reversible! Summary Thermal expansion 𝐿 = 𝐿0 (1 + 𝛼Δ𝑇) Ideal gas law 𝑝𝑉 = 𝑛𝑅𝑇 van der Waals equation 𝑝− 𝑛 2𝑎 𝑉 = 𝑉0 (1 + 𝛽Δ𝑇) 𝑉 − 𝑛𝑏 = 𝑛𝑅𝑇 𝑉2 Avogadro’s law 𝑉 = 𝑛 𝑉𝑚 Heat 𝑄 = 𝑐𝑚 𝑛∆𝑇 = 𝑐𝑠 𝑚∆𝑇 Work (volume) 𝑑𝑊 = −𝑝𝑑𝑉 Dalton’s law 𝑝= 𝑖 𝑝𝑖 = 𝑖 𝑛 𝑖 𝑅𝑇 𝑉 𝜚 = 𝜚0 (1 − 𝛽Δ𝑇)