* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 3 - CFD - Anna University
Dynamic insulation wikipedia , lookup
Equipartition theorem wikipedia , lookup
Calorimetry wikipedia , lookup
Thermoregulation wikipedia , lookup
Temperature wikipedia , lookup
Thermal conductivity wikipedia , lookup
Heat capacity wikipedia , lookup
Heat exchanger wikipedia , lookup
Conservation of energy wikipedia , lookup
Thermal radiation wikipedia , lookup
Heat equation wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Internal energy wikipedia , lookup
Countercurrent exchange wikipedia , lookup
R-value (insulation) wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat transfer wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermal conduction wikipedia , lookup
Adiabatic process wikipedia , lookup
Thermodynamic system wikipedia , lookup
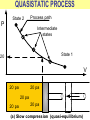
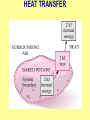
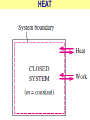
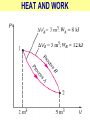
ENGINEERING THERMODYNAMICS Dr. M.R.SWAMINATHAN Assistant Professor Internal Combustion Engineering Division Department of Mechanical Engineering ANNA UNIVERSITY CHENNAI-25. PROPERTIES- contd. CYCLE A process (or a series of connected processes) with identical end states is called a cycle. A cycle composed of two processes, A and B. all other thermodynamic properties must also change Other thermodynamic properties must also change so that the pressure is a function of volume as described by these two processes 2 P Process B 1 Process A V EQUILIBRIUM A system is said to be in thermodynamic equilibrium if it maintains Thermal (Uniform Temperature) Mechanical (Uniform Pressure) Phase equilibrium Chemical equilibrium. 20 °C 30 °C 30 °C 35 °C 32 °C 32 °C 32 °C 40 °C No thermal equilibrium 32 °C 32 °C Thermal equilibrium QUASISTATIC PROCESS Process proceeds in such a way that the system remains infinitesimally close to an equilibrium state at all times, it is called a quasistatic, or quasi-equilibrium process QUASISTATIC PROCESS Quasi- equilibrium process can be viewed as a sufficiently slow process that allows the system to adjust itself internally so that properties in one part of the system do not change any faster than those at other parts QUASISTATIC PROCESS QUASISTATIC PROCESS State 2 P Process path Intermediate states State 1 20 V 20 pa 20 pa 20 pa 20 pa 20 pa (a) Slow compression (quasi-equilibrium) ZEROTH LAW Zeroth law was first formulated and labeled by R. H. Fowler in 1931 • Two bodies are in thermal equilibrium if both have the same temperature even if they are not in contact. HEAT • Heat is defined as the form of energy that is transferred between two systems (or a system and its surroundings) by virtue of a temperature difference. • Heat is energy in transition. HEAT • Heat is energy in transition. It is recognized only as it crosses the boundary of a system • The potato contains energy, but this energy is heat transfer only as it passes through the skin of the potato (the system boundary) to reach the air, as shown in figure. HEAT TRANSFER HEAT HEAT INTERNAL ENERGY • Energy possessed by the molecules by the virtue of its temperature • Higher the temperature higher is the internal energy possessed by the medium ( solid / liquid/ gas ) • Internal energy is zero at absolute zero INTERNAL ENERGY-contd. • Internal energy is the sum of all microscopic forms of energy of a system. • Internal energy will be highest for gas phase and minimum for the solid phase THERMODYNAMIC PROCESSES • There are many thermodynamic processes in practice. • In each of the processes we normally allow one of the properties to remain a constant during a process. THERMODYNAMIC PROCESSES • • • • • • Isobaric Isochoric Isothermal Polytropic Adiabatic Isentropic (P=c) (V=c) (T=c) (PVn=c) (PVγ=c) (PVγ=c) WORK • Work, like heat, is an energy interaction between a system and its surroundings. • Energy can cross the boundary of a closed system in the form of heat or work. WORK • Work is the energy transfer associated between a system & the surrounding in the absence of any temperature difference. • An example is the work done in expansion of a piston assuming there is no temperature difference between the cylinder and surroundings WORK contd. • • • • Displacement work (pdV work) Force exerted, F= p. A Work done dW= F.dL= p. A dL= p.dV If the piston moves through a finite distance say 1-2,Then work done has to be evaluated by integrating dW= ∫pdV DISPLACEMENT WORK FLOW WORK • Open systems involves flow of mass in and out of the system unlike closed system • Energy associated with pushing mass or volume of a gas in or out of the system is called FLOW WORK or FLOW ENERGY FLOW WORK contd. • FLOW WORK is always associated with open systems or a stream of liquid or gas which is in motion • Flow work is given by p1v1- p2v2 or simply as pv TYPES OF WORK • • • • • • Stretching of a wire Electrical Energy Work of a reversible chemical cell Work in stretching of a liquid surface Work done on elastic solids Work of polarization and magnetization HEAT & WORK TRANSFER • All our efforts are oriented towards how to convert heat to work or vice versa: • Heat to work • Work to heat Thermal power plant Refrigeration HEAT AND WORK • Heat and work are directional quantities. WORK INTERACTION • Work done by a system or work given by a system is considered + ve e.g work supplied by turbine • Work done on the system or work supplied to system is considered –ve e.g compressor or pump HEAT INTERACTION • Heat supplied to the system is considered + ve e.g heat supplied to boil water in a boiler • Heat rejected by a system is considered – ve e.g condenser of a thermal power plant HEAT AND WORK POINT & PATH FUNCTIONS • Both heat and work are path functions • They depend only on the path of travel and not on end states or end points • BOTH HEAT AND WORK ARE PATH FUNCTIONS (INEXACT DIFFERENTIAL) POINT & PATH FUNCTIONS • Properties like pressure , temperature depend only on end state • They are POINT FUNCTIONS & are known as EXACT DIFFERENTIALS. • All Exact differentials are property of a system HEAT & WORK SIMILARITY • Heat and work are energy transfer mechanisms between a system and its surroundings. • Systems possess energy, but not heat or work. • Both are recognised at the boundaries of a system as they cross the boundaries. • Both are path functions WORK INTERACTION Consider following example the An electric oven heat by a heating element HEAT INTERACTION • Consider the same example • An electric oven heat by a heating element







































![[A, 8-9]](http://s1.studyres.com/store/data/006655537_1-7e8069f13791f08c2f696cc5adb95462-150x150.png)