* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bagian 2 termodinamika
Insulated glazing wikipedia , lookup
Internal energy wikipedia , lookup
Heat capacity wikipedia , lookup
Temperature wikipedia , lookup
Thermal radiation wikipedia , lookup
Calorimetry wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Maximum entropy thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Adiabatic process wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
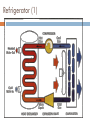
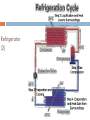
KIMIA LINGKUNGAN BAGIAN 2: TERMODINAMIKA PREVIEW In this third part of the course we: define and apply a number of thermodynamic ideas and concepts become familiar with and apply the 1st and 2nd law of thermodynamics discuss several thermodynamic processes with the aid of pressure-volume (pV) diagrams define and discuss the concept of entropy apply the laws of thermodynamics to discuss heat engines, refrigerators and heat pumps introduce parameters to quantify the efficiency at which thermodynamic devices operate learn about the Carnot cycle and its relation to the concept of an 'ideal' engine Thermodynamic Systems, States and Processes Objectives are to: define thermodynamics systems and states of systems explain how processes affect such systems apply the above thermodynamic terms and ideas to the laws of thermodynamics Thermodynamic Systems A thermodynamic system is a collection of matter which has distinct boundaries. OR A real or imaginary portion of universe whish has distinct boundaries is called system. OR A thermodynamic system is that part of universe which is under thermodynamic study. 1. 1st Law of Thermodynamics U Q W positive Q : heat added to system positive W : work done by system statement of energy conservation for a thermodynamic system internal energy U is a state variable W, Q process dependent Isoprocesses apply 1st law of thermodynamics to closed system of an ideal gas isoprocess is one in which one of the thermodynamic (state) variables are kept constant use pV diagram to visualise process Isobaric Process process in which pressure is kept constant Isochoric Process process in which volume is kept constant Isothermal Process process in which temperature is held constant Adiabatic Process process in which no heat transfer takes place 2. Second Law of Thermodynamics and Entropy Objectives are to: state and explain the second law of thermodynamics explain the concept of entropy 2nd Law of Thermodynamics states in which direction a process can take place heat does not flow spontaneously from a cold to a hot body heat cannot be transformed completely into mechanical work it is impossible to construct an operational perpetual motion machine introduces concept of entropy Entropy property that indicates the direction of a process entropy is a measure of disorder entropy is a measure of a system’s ability to do useful work entropy determines direction of time the entropy of an isolated system increases Q S (change in entropy at constant t emperature ) T 2nd Law of Thermodynamics: entropy 2nd Law example 3. Heat Engines and Heat Pumps Objectives are to: explain what a heat engine is, and compute its thermal efficiency explain what a heat pump is, and compute its coefficient of performance Diagram of a Heat Engine Heat Engine Heat Engine A heat engine is a cyclic device that converts thermal energy into work output It is a device that takes heat from a high-T reservoir, converts some of to (useful) work, and transfers the rest to the surroundings (a low-T reservoir) Examples: steam engines; internal combustion engines (car engines) Thermal efficiency (“what you get out/what you put in”): Qc work out Wout eth 1 heat in Qin Qh No heat engine operating in a cycle can convert all of its heat input completely to work Heat Pump Heat Pump A heat pump is a (cyclic) device that transfers heat energy from a low-T reservoir to a high-T reservoir Examples: air conditioner; refrigerator Coefficient of performance (“what you get out/what you put in”): cophp Qc heat transfer Qin work done W Qh Qc No heat pump operating in a cycle can transfer thermal energy to a low-T reservoir without doing some work Refrigerator (1) Refrigerator (2) 4. The Second Law Revisited •it is impossible to produce a cyclic engine that generates work by extracting heat from a reservoir without expelling some waste heat •it is impossible to produce a heat pump in which the sole result is the transfer of heat from a lowT to a high-T body 5. Third Law of Thermodynamics The 3rd law states that: It is impossible to reach a temperature of absolute zero It is impossible to have a (Carnot) efficiency equal to 100% (this would imply Tc = 0). Isobaric Expansion: Change in Internal Energy A quantity of an ideal gas has a volume of 22.4 litres at STP (standard temperature and pressure). While absorbing 315 cal of heat from the surroundings, the gas expands isobarically to 32.4 litres. What is the change in internal energy of the gas? What is the equilibrium temperature (in degrees Celsius) of the gas after expansion? Question Three different experiments are run, in which a gas expands from point A to point D along the three paths shown below. Calculate the amount of work done for paths 1, 2 and 3. Questions Free Loader: Consider the following idea. A ship heats its boilers and propels itself without the use of coal or oil in the following way. It pumps in warm sea water, extracts heat from that sea water, concentrates the extracted heat in its boilers, and discharges the cooled seawater back into the ocean. The discharged water may be ice if enough heat has been taken from it. Could this idea be made to work? Gulf of Mexico: Another free loader idea is to generate power as follows. Water on top of the Gulf of Mexico is quite warm but deep down the water is cold. The plan is to heat some gas with warm water from the top so it will expand, and then cool the gas with water from the bottom so it will contract. The gas is alternately expanded and contracted so it drives a piston back and forth. The moving piston is attached by conventional means to an electric generator to make electricity. Can this idea be made to work?