Glossary - WordPress.com
... A solution in which one mole of a solute has been dissolved in one dm3 of solution. It is represented as M. Metallic Bond When positively charged metal ions are held together by freely moving electrons, the bond formed is called a metallic bond. Molecular Solid A solid which has Vander Waal’s forces ...
... A solution in which one mole of a solute has been dissolved in one dm3 of solution. It is represented as M. Metallic Bond When positively charged metal ions are held together by freely moving electrons, the bond formed is called a metallic bond. Molecular Solid A solid which has Vander Waal’s forces ...
Exam 3 Answer Key
... All NO3-, C2H3O2-, ClO3-, and C1O4- salts are soluble. All Ag+, Pb2+ , and Hg22+ salts are insoluble. All Cl- , Br- , and I- salts are soluble. All CO32-, O2-, S2-, OH-, SO32-, CrO42-, Cr2O72-, and PO43salts insoluble, except CaS, SrS, BaS and Ba(OH)2. All SO42- salts are soluble except Ca2+, Sr2+, ...
... All NO3-, C2H3O2-, ClO3-, and C1O4- salts are soluble. All Ag+, Pb2+ , and Hg22+ salts are insoluble. All Cl- , Br- , and I- salts are soluble. All CO32-, O2-, S2-, OH-, SO32-, CrO42-, Cr2O72-, and PO43salts insoluble, except CaS, SrS, BaS and Ba(OH)2. All SO42- salts are soluble except Ca2+, Sr2+, ...
Electronic excitation gives informative fragmentation of polypeptide
... techniques. Only a few studies that report on tandem mass spectrometry (MS/MS) of deprotonated peptides larger than ...
... techniques. Only a few studies that report on tandem mass spectrometry (MS/MS) of deprotonated peptides larger than ...
PowerPoint
... TOF Coincidence map for Ar8+ + N2 products. a) – conventional mode (fragment ions are detected on PSD), b) – ZOO-RISE mode (secondary electrons are detected on PSD). ...
... TOF Coincidence map for Ar8+ + N2 products. a) – conventional mode (fragment ions are detected on PSD), b) – ZOO-RISE mode (secondary electrons are detected on PSD). ...
Spectra
... 12C. Likewise there are isotopes of hydrogen including 1H, 2H and 3H. Hence a fragment with the formula CH3 has a a predominate mass of 15 but has smaller peaks representing the heavier isotopes of carbon ...
... 12C. Likewise there are isotopes of hydrogen including 1H, 2H and 3H. Hence a fragment with the formula CH3 has a a predominate mass of 15 but has smaller peaks representing the heavier isotopes of carbon ...
Water Chemistry - Biology12-Lum
... Although a water molecule has an overall neutral charge (having the same number of electrons and protons), the electrons are asymmetrically distributed, which makes the molecule polar. The oxygen nucleus draws electrons away from the hydrogen nuclei, leaving these nuclei with a small net positive ch ...
... Although a water molecule has an overall neutral charge (having the same number of electrons and protons), the electrons are asymmetrically distributed, which makes the molecule polar. The oxygen nucleus draws electrons away from the hydrogen nuclei, leaving these nuclei with a small net positive ch ...
Nugget
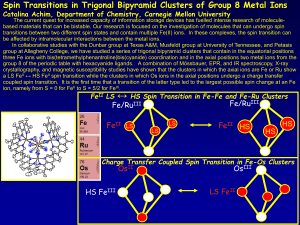
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
Chem Ch 4 test review
... What is a period? Identify elements on the periodic table by their periods or groups. What do elements in groups have in common? Why? 9. Describe the natural states of the elements, i.e., which are solids, liquids, or gases at around 25oC. What is a diatomic molecule? Which elements are diatomic mol ...
... What is a period? Identify elements on the periodic table by their periods or groups. What do elements in groups have in common? Why? 9. Describe the natural states of the elements, i.e., which are solids, liquids, or gases at around 25oC. What is a diatomic molecule? Which elements are diatomic mol ...
Ch 6 Jeopardy Review
... with atomic numbers close to helium, this is how many electrons are needed to fill the outermost energy level. ...
... with atomic numbers close to helium, this is how many electrons are needed to fill the outermost energy level. ...
VSPER, Molecular Orbitals, and Organic Molecules
... • They each have strengths and weaknesses with respect to what they are able to show and what the need the viewer to assume in order to understand their meaning • For example, Lewis dot diagrams are very clear at showing which electrons are principally associated with which atoms • However, as we no ...
... • They each have strengths and weaknesses with respect to what they are able to show and what the need the viewer to assume in order to understand their meaning • For example, Lewis dot diagrams are very clear at showing which electrons are principally associated with which atoms • However, as we no ...
Study Guide for Exam 2_Sp12
... What is the difference between molecular mass and formula mass? You should be able to use the periodic table and a chemical formula to calculate the molecular mass or the formula mass of any compound or element. You need to memorize Avogadro’s number. What is a mole? You should be able to use Avogad ...
... What is the difference between molecular mass and formula mass? You should be able to use the periodic table and a chemical formula to calculate the molecular mass or the formula mass of any compound or element. You need to memorize Avogadro’s number. What is a mole? You should be able to use Avogad ...
Trends in the Periodic Table
... movement of the subatomic particles, specifically electrons? • A: Temperature does not have enough energy to affect subatomic particle movement, only molecule movement. ...
... movement of the subatomic particles, specifically electrons? • A: Temperature does not have enough energy to affect subatomic particle movement, only molecule movement. ...
Worksheet on Ionic and Atomic Size Trends
... levels, the greater nuclear charge in chlorine makes the atom smaller. 12. The sodium ion is smaller than the atom, because when sodium loses its valence electron to form an ion, it also loses its 3rd energy level. 13. The chlorine ion is larger than the chlorine atom, because adding an additional e ...
... levels, the greater nuclear charge in chlorine makes the atom smaller. 12. The sodium ion is smaller than the atom, because when sodium loses its valence electron to form an ion, it also loses its 3rd energy level. 13. The chlorine ion is larger than the chlorine atom, because adding an additional e ...
Extended Abstract Template
... Solid phase extraction (SPE) is a widely used extraction method in sample preparation and pre-concentration because of high concentration factor. It is normally performed using either cartridge or disc format. However, commercially available SPE sorbent is relatively expensive and SPE process is les ...
... Solid phase extraction (SPE) is a widely used extraction method in sample preparation and pre-concentration because of high concentration factor. It is normally performed using either cartridge or disc format. However, commercially available SPE sorbent is relatively expensive and SPE process is les ...
On the Modeling of the Production and Drift of Carriers in
... model can reproduce the Trichel pulses measured when a voltage of 2500 V is applied to a 1 μm needle point [5]. Considering the discussion in Section II.B, the simulations consider field emission and impact ionization only. As it can be seen in Fig. 6, the simulated current in the configuration once ...
... model can reproduce the Trichel pulses measured when a voltage of 2500 V is applied to a 1 μm needle point [5]. Considering the discussion in Section II.B, the simulations consider field emission and impact ionization only. As it can be seen in Fig. 6, the simulated current in the configuration once ...
Trends in the Periodic Table
... movement of the subatomic particles, specifically electrons? • A: Temperature does not have enough energy to affect subatomic particle movement, only molecule movement. ...
... movement of the subatomic particles, specifically electrons? • A: Temperature does not have enough energy to affect subatomic particle movement, only molecule movement. ...
Chapter 3
... • Atoms- the smallest particles that make up all matter – Protons(+): positively charged particles found in the nucleus of an atom – Neutrons(o): neutral particles found in the nucleus of an atom – Electrons(-): negatively charged particles found outside of the nucleus ...
... • Atoms- the smallest particles that make up all matter – Protons(+): positively charged particles found in the nucleus of an atom – Neutrons(o): neutral particles found in the nucleus of an atom – Electrons(-): negatively charged particles found outside of the nucleus ...
Culver City H.S. • AP Chemistry Name Period ___ Date ___/___/___
... An electron is excited from the n=1 ground state to the n=3 state in a hydrogen atom. Which of the following statements are true? Correct the false statements to make them true. It takes more energy to ionize (completely remove) the electron from n=3 than from the ground state. The electron is farth ...
... An electron is excited from the n=1 ground state to the n=3 state in a hydrogen atom. Which of the following statements are true? Correct the false statements to make them true. It takes more energy to ionize (completely remove) the electron from n=3 than from the ground state. The electron is farth ...
Chapter 8 Notes
... Lattice energy increases with decreasing ionic radii. This makes sense if you think about it. After-all the smaller the ion, the closer the positive nucleus is to the valence electrons responsible for bonding. So Magnesium compounds will have higher (negative) lattice energies than Calcium compounds ...
... Lattice energy increases with decreasing ionic radii. This makes sense if you think about it. After-all the smaller the ion, the closer the positive nucleus is to the valence electrons responsible for bonding. So Magnesium compounds will have higher (negative) lattice energies than Calcium compounds ...
Solution
... For the following ten questions, consider the lowest energy Lewis structure (minimized formal charge etc.) for the following molecules/ions: XeF4, XeO4, OCN–1 (you may want to draw the Lewis structures in the space provided, the central atom is highlighted). ...
... For the following ten questions, consider the lowest energy Lewis structure (minimized formal charge etc.) for the following molecules/ions: XeF4, XeO4, OCN–1 (you may want to draw the Lewis structures in the space provided, the central atom is highlighted). ...
FORM 1 GEOGRAPHY REVISION GRID
... State that during a chemical change a new substance is made Recall the differences between a chemical and a physical change ...
... State that during a chemical change a new substance is made Recall the differences between a chemical and a physical change ...
CERN workshop 2015
... Göran Andersson (one of the founders of ISOLDE) retired and was succeeded by Björn Jonson Björn gave me the isotope separator LILLJON I could start investigating negative ions ...
... Göran Andersson (one of the founders of ISOLDE) retired and was succeeded by Björn Jonson Björn gave me the isotope separator LILLJON I could start investigating negative ions ...
ATOM
... Mass Spectrometer • Obtain atomic and molecular weights • Need gaseous samples • Ionization of sample by high energy electrons • Pass gaseous ions through poles of a magnet • For ions carrying the same charge, more massive particles will be less deflected than the less massive particles. Thus, mass ...
... Mass Spectrometer • Obtain atomic and molecular weights • Need gaseous samples • Ionization of sample by high energy electrons • Pass gaseous ions through poles of a magnet • For ions carrying the same charge, more massive particles will be less deflected than the less massive particles. Thus, mass ...
Ion source

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.