Ion mobility for explosives and drugs for microchip FAIMS
... New method The conditions for a μ-FAIMS system are very different from that of large dimensional cylindrical or planar FAIMS systems. First the channels are much narrower, and the electric field is much higher than those in the conventional cylindrical or planar FAIMS. Second, the low part of the cy ...
... New method The conditions for a μ-FAIMS system are very different from that of large dimensional cylindrical or planar FAIMS systems. First the channels are much narrower, and the electric field is much higher than those in the conventional cylindrical or planar FAIMS. Second, the low part of the cy ...
NOMENCLATURE OF IONIC COMPOUNDS CHEMISTRY 1411
... One may be tempted to put the oxidatiion number of Barium within parenthesis such as Barium (II) and this is incorrect. Oxidation number is expressed in parenthesis only for transistion metal ions or metal ions which show variable oxidation numbers. Barium belongs to group 2 and all elements in grou ...
... One may be tempted to put the oxidatiion number of Barium within parenthesis such as Barium (II) and this is incorrect. Oxidation number is expressed in parenthesis only for transistion metal ions or metal ions which show variable oxidation numbers. Barium belongs to group 2 and all elements in grou ...
72KB
... H2O – weak force of attraction circled (force between adjacent molecules). NaCl – ionic bond circled (force between positive and negative ions). Sodium and chloride ions are held together by strong ionic bonds. A large amount of energy is required to overcome these forces. Hence NaCl has a high melt ...
... H2O – weak force of attraction circled (force between adjacent molecules). NaCl – ionic bond circled (force between positive and negative ions). Sodium and chloride ions are held together by strong ionic bonds. A large amount of energy is required to overcome these forces. Hence NaCl has a high melt ...
Northgate High School Chemistry Department
... describe the term covalent bond as a shared pair of electrons; construct ‘dot-and-cross’ diagrams to describe: (i) single covalent bonding, eg as in H2, Cl2, HCl, H2O, NH3, CH4, BF3 and SF6, (ii) multiple covalent bonding, eg as in O2, N2 and CO2, (iii) dative covalent (coordinate) bonding, eg as in ...
... describe the term covalent bond as a shared pair of electrons; construct ‘dot-and-cross’ diagrams to describe: (i) single covalent bonding, eg as in H2, Cl2, HCl, H2O, NH3, CH4, BF3 and SF6, (ii) multiple covalent bonding, eg as in O2, N2 and CO2, (iii) dative covalent (coordinate) bonding, eg as in ...
Electrochemistry 2
... Corrosion is a process where materials are gradually destroyed by chemical reac)ons. The most common form of corrosion is the oxida)on of metals by oxygen — we are especially familiar with the case o ...
... Corrosion is a process where materials are gradually destroyed by chemical reac)ons. The most common form of corrosion is the oxida)on of metals by oxygen — we are especially familiar with the case o ...
7B35.75 Plasma Tubes
... A plasma ball consists of a small electrode inside a low pressure glass dome containing a mixture of inert gases, such as argon and neon. A low pressure is necessary so that the gases can be ionized easier, and inert gases must be used so that there is no reaction between the gas and the metal elect ...
... A plasma ball consists of a small electrode inside a low pressure glass dome containing a mixture of inert gases, such as argon and neon. A low pressure is necessary so that the gases can be ionized easier, and inert gases must be used so that there is no reaction between the gas and the metal elect ...
Test 4 Review - Ralph C. Mahar
... The metal must be above (more active than) the ion for it to be a spontaneous reaction. ...
... The metal must be above (more active than) the ion for it to be a spontaneous reaction. ...
Dynamics and particle uxes in atmospheric
... interest of microplasmas for most applications. Here we show that microdischarges offer not only interesting dynamics but also a route to efficient delivery of both neutral and charged species. He+H2O plasmas are known to produce valuable ROS9, 19 such as OH and H2O2 that are of interest for biomedi ...
... interest of microplasmas for most applications. Here we show that microdischarges offer not only interesting dynamics but also a route to efficient delivery of both neutral and charged species. He+H2O plasmas are known to produce valuable ROS9, 19 such as OH and H2O2 that are of interest for biomedi ...
How many molecules?
... (a string of these reactions is a decay chain) • The rates of each decay are variable – some are extremely slow • If a system is closed (no elements escape) then the proportion of parent (original) and daughter (product of a radioactive decay reaction) can yield a date. • Radioactive isotopes are al ...
... (a string of these reactions is a decay chain) • The rates of each decay are variable – some are extremely slow • If a system is closed (no elements escape) then the proportion of parent (original) and daughter (product of a radioactive decay reaction) can yield a date. • Radioactive isotopes are al ...
CNT Sensors for Detecting Gases with Low Adsorption Energy by Ionization
... Early chemical types of CNT sensors were reported in some journals [9-12]. They usually figured out the data with the changes of conductivity and permittivity in CNTs by gas adsorption, and have shown merits such as good electrical response even at room temperature as well as a small size. On the ot ...
... Early chemical types of CNT sensors were reported in some journals [9-12]. They usually figured out the data with the changes of conductivity and permittivity in CNTs by gas adsorption, and have shown merits such as good electrical response even at room temperature as well as a small size. On the ot ...
Carbon–carbon bond cleavage in the photoionization of ethanol and
... The alcohols are one of the systems that have been shown to have these properties. The products of ion–molecule reactions within the alcohol cluster ions are very similar to the products from the gas phase. Due to the large cross section of proton transfer reaction in the alcohols, the protonated al ...
... The alcohols are one of the systems that have been shown to have these properties. The products of ion–molecule reactions within the alcohol cluster ions are very similar to the products from the gas phase. Due to the large cross section of proton transfer reaction in the alcohols, the protonated al ...
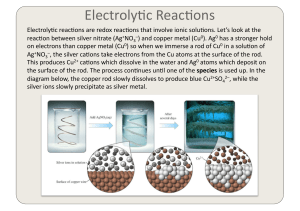
Oxidation number and Electrolysis(電解)
... also give out electrons to form metal ions, i.e., the metal electrode may compete with the anion in giving out electrons. For example, if copper electrode is used as the anode in the electrolysis of copper sulfate solution, the copper electrode becomes thinner and thinner. ...
... also give out electrons to form metal ions, i.e., the metal electrode may compete with the anion in giving out electrons. For example, if copper electrode is used as the anode in the electrolysis of copper sulfate solution, the copper electrode becomes thinner and thinner. ...
Evaporation–glow discharge hybrid source for plasma immersion
... Fig. 2 is a picture depicting the glow discharge of the hybrid plasma source. Some of the typical characters of glow discharge are noticeable. The brightest part of the discharge is different from the normal parallel plane electrode glow discharge where the brightest part is usually the negative glo ...
... Fig. 2 is a picture depicting the glow discharge of the hybrid plasma source. Some of the typical characters of glow discharge are noticeable. The brightest part of the discharge is different from the normal parallel plane electrode glow discharge where the brightest part is usually the negative glo ...
JF CH 1101 General and Physical Chemistry 2013
... incompletely formed in front of the moving ion, and incompletely decayed behind the moving ion. The net result is the displacement of the centre of charge of the atmosphere behind the moving ion. Because the charge on the ion and the atmosphere are opposite, the result is a retardation in the motion ...
... incompletely formed in front of the moving ion, and incompletely decayed behind the moving ion. The net result is the displacement of the centre of charge of the atmosphere behind the moving ion. Because the charge on the ion and the atmosphere are opposite, the result is a retardation in the motion ...
TDR XFEL workshop series Atomic, molecular and cluster physics
... Trapped Ion X-Ray Diffraction ...
... Trapped Ion X-Ray Diffraction ...
Chpt2 - Dr. Erdal ONURHAN
... o All atoms of the same element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. o Compounds are composed of atoms of more than one element. In any compound, the ratio of numbers of atoms pf any of the two ...
... o All atoms of the same element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. o Compounds are composed of atoms of more than one element. In any compound, the ratio of numbers of atoms pf any of the two ...
Request reprint © - Research at the Department of Chemistry
... quadrupole and mass analyzed to obtain a surfaceinduced dissociation (SID) spectrum. Because the dinuclear complexes of interest are doubly charged, the peaks in the mass spectrum were separated by 0.5 u. The entire envelope of isotopes of the precursor ion was selected and allowed to collide with t ...
... quadrupole and mass analyzed to obtain a surfaceinduced dissociation (SID) spectrum. Because the dinuclear complexes of interest are doubly charged, the peaks in the mass spectrum were separated by 0.5 u. The entire envelope of isotopes of the precursor ion was selected and allowed to collide with t ...
Final Exam A - Answers - San Diego Chemistry Tutor
... 27. Water boils more easily (at lower temperatures) at higher altitudes than it does at sea level. Which factor below best explains why this happens? a) This is a colligative property of water. b) Temperatures cannot be properly measured at higher altitudes. c) The vapor pressure of water increases ...
... 27. Water boils more easily (at lower temperatures) at higher altitudes than it does at sea level. Which factor below best explains why this happens? a) This is a colligative property of water. b) Temperatures cannot be properly measured at higher altitudes. c) The vapor pressure of water increases ...
23.2 - Transition-Metal Complexes 23.3
... 4) Use pre xes to denote the number of simple ligands (if the name of the ligands already has such a pre x, use alternate pre xes of bis, tris, tetrakis, etc.) 5) For complex anions, add -ate to the Latin name of the metal 6) Give the oxidation number of the metal *In complex anions (negative charge ...
... 4) Use pre xes to denote the number of simple ligands (if the name of the ligands already has such a pre x, use alternate pre xes of bis, tris, tetrakis, etc.) 5) For complex anions, add -ate to the Latin name of the metal 6) Give the oxidation number of the metal *In complex anions (negative charge ...
KINETIC ENERGY DISTRIBUTION OF IONS GENERATED BY
... Hamlling of the transient recorder was carried out by the fast handshake capability of the IEEE-488 interface that facilitated fast data transfer between peripheral and computer at about 100 kbyte s-l rate. Handhg of the other devices was carried out with the powerful data acquisition system of the ...
... Hamlling of the transient recorder was carried out by the fast handshake capability of the IEEE-488 interface that facilitated fast data transfer between peripheral and computer at about 100 kbyte s-l rate. Handhg of the other devices was carried out with the powerful data acquisition system of the ...
View accepted manuscript: Metastable triply charged diatomic
... In the tunneling limit [1] of multiphoton atomic ionization, the laser field oscillates so slowly that electrons adiabatically adjust to the field. Only the most weakly bound electron can tunnel through the oscillating barrier created by the laser field and the binding potential. Together with the h ...
... In the tunneling limit [1] of multiphoton atomic ionization, the laser field oscillates so slowly that electrons adiabatically adjust to the field. Only the most weakly bound electron can tunnel through the oscillating barrier created by the laser field and the binding potential. Together with the h ...
Hydrocarbon ions in fuel-rich, CH4-C2H2-0, flames
... Most commonly, the product of this reaction is a mentioned in the previous section, namely, chemistable (i.e. non-radical) hydrocarbon molecule. ionization and thermal ionization. If the pair of Associative detachment reactions are also ex- curves with Tad= 2401 and 2488 K for $ = 2.22 f pected to b ...
... Most commonly, the product of this reaction is a mentioned in the previous section, namely, chemistable (i.e. non-radical) hydrocarbon molecule. ionization and thermal ionization. If the pair of Associative detachment reactions are also ex- curves with Tad= 2401 and 2488 K for $ = 2.22 f pected to b ...
Proteomics1_2012
... 1. Ionize (i.e. charge) peptide fragments 2. Separate ions by mass/charge (m/z) ratio 3. Detect ions of different m/z ratio 4. Compare to database of predicted m/z fragments for each genome ...
... 1. Ionize (i.e. charge) peptide fragments 2. Separate ions by mass/charge (m/z) ratio 3. Detect ions of different m/z ratio 4. Compare to database of predicted m/z fragments for each genome ...
Ion source

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.