* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrochem 1 - GCG-42
Acid–base reaction wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Electron scattering wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Membrane potential wikipedia , lookup
Heat transfer physics wikipedia , lookup
Marcus theory wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Ionic liquid wikipedia , lookup
Thermal conductivity wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Ionic compound wikipedia , lookup
Electrochemistry wikipedia , lookup
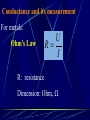
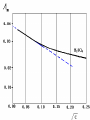
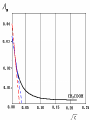
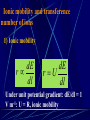
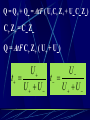
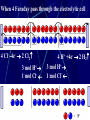
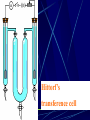
Electrochemistry -1 Puneet Jyoti G.C.G-42 Chd. Electron Transfer Reactions Results in the generation of an electric current (electricity) or be caused by imposing an electric current. Electron transfer reactions are oxidation-reduction or redox reactions. Therefore, this field is often called ELECTROCHEMISTRY. Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (a metal or a semiconductors) and an ionic conductor (the electolyte), and which involve electron transfer between the electrode and the electrolyte or species in solution. Motion of ions in the solution: 1) diffusion: due to difference in concentration 2) convection: due to the difference in density or temperature 3) transfer: due to electric field Only the transfer can cause net electricity Conductance and its measurement For metals: Ohm’s Law U R I R: resistance Dimension: Ohm, Resistivity l R A Dimension: Ohm m, m For electrolytic solution: electric conductance (G) : Definition: G = 1/R Dimension: -1, mho, Siemens, S conductivity () or spedific conductance: Definition: = 1/ Dimension: S m-1 Cell constant of a conductivity cell l G K cell G A K cell R Influential factors for conductivity 1) concentration – dependence of conductance 1. Acids and bases have higher conductance 2. C < 5 mol dm-3, increases with C 3. For CH3COOH conductance does not depend on C (2) temperaturedependence of conductance Molar conductivity The conductivity of a solution is approximately proportional to the concentration 1) Definition m C m 1 V V V: degree of dilution m is the conductivity contributed by 1 mole of electrolyte between electrodes of 1 m apart Dependence of molar conductivity on concentration m decreases with concentration. Due to the interaction between ions: interionic attraction Kohlrausch replotted m against C1/2 For 1:1 electrolytes: C < 0.002~ 0.003 mol dm-3 Linear relationship between m and C1/2 can be observed. Kohlrausch empirical formula m m A c To extrapolate the linear part of m ~ C1/2 at low concentration to C = 0, m can be obtained. m the limiting value of m at infinite dilution: limiting molar conductivity Kohlrausch’s law of independent ionic mobilities m m, m, At infinite dilution, m should be the sum of the separate contributions of the ions limiting molar conductivity of weak electrolyte ( HAc ) ( H ) ( Ac ) m m m ( H ) (Cl ) ( Na ) m m m ( Ac ) ( Na ) (Cl ) m m m m m m ( HCl ) ( NaAc) ( NaCl ) Ionic mobility and transference number of ions 1) Ionic mobility dE r dl dE r U dl Under unit potential gradient: dE/dl = 1 V m-1: U = R, ionic mobility 2) Transference number I = I + + IQ = Q + + Q- tj Qj Q The fraction of the current transported by an ion is its transference number or transport number t = t + + t- = 1 3) Relation between ionic mobility and transference number C-, Z-, U-; C+, Z+, U+; For time t: Q+ = A U+t C+ Z+ F Q = A Ut C Z F Q = Q+ + Q = AtF ( U+C+ Z+ + U C Z) C+ Z+ = C Z Q = AtF C+ Z+ ( U+ + U) U U t t U U U U Measurement of transference numbers 1) Hittorf method (1853) Electrolysis of HCl solution Anodic region Bulk solution cathodic region When 4 Faraday pass through the electrolytic cell 4 Cl- -4e- 2 Cl2 3 mol H+ 1 mol Cl- 4 H+ +4e- 2 H2 3 mol H+ 1 mol Cl- For anodic region: Cresidual = Cinitial – Creact + C transfer 3 = 6 – t- = 1 / 4 = 0.25 4 + C transfer t+ = 3 / 4 = 0.75 Hittorf’s transference cell 2) The moving-boundary method MA, MA’ have an ion in common. The boundary, rather difference in color, refractivity, etc. is sharp. In the steady state, the two ions move with the same velocity. When Q coulomb passes, the boundary moves x, the crosssectional area of the tube is A: xACZ+F = t+Q THANX