* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrochemistry 2
Metastable inner-shell molecular state wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Electron configuration wikipedia , lookup
Chemical bond wikipedia , lookup
Ionic compound wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Atomic theory wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
History of electrochemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Microbial fuel cell wikipedia , lookup
Electrochemistry wikipedia , lookup
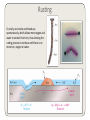
Electroly)c Reac)ons Electroly)c reac)ons are redox reac)ons that involve ionic solu)ons. Let’s look at the reac)on between silver nitrate (Ag+NO3–) and copper metal (Cu0). Ag0 has a stronger hold on electrons than copper metal (Cu0) so when we immerse a rod of Cu0 in a solu)on of Ag+NO3–, the silver ca)ons take electrons from the Cu atoms at the surface of the rod. This produces Cu2+ ca)ons which dissolve in the water and Ag0 atoms which deposit on the surface of the rod. The process con)nues un)l one of the species is used up. In the diagram below, the copper rod slowly dissolves to produce blue Cu2+SO42–, while the silver ions slowly precipitate as silver metal. Ionic and Half Equa)ons Now let’s take a look at the equa)ons for this reac)on: The full equa)on for this reac)on is: Cu0 + 2Ag+(NO3)– Cu2+(NO3)–2 + 2Ag0 We can see that the NO3– ion does not change in this reac)on. In fact, it is not important to the reac)on and is only a spectator ion (we first saw spectator ions in acid/base chemistry). In this case, an ionic equa,on, where we only look at the ions that change oxida)on state, may be clearer. The ionic equa)on for this reac)on is: 2Ag+ + Cu0 Cu2+ + 2Ag0 Since, in electrochemistry and redox reac)ons, we are interested in the flow of electrons, it is also useful to make half-‐equa)ons. Half equa)ons only look at one ion at a )me and how it gains or loses electrons. We have to balance the two half equa)ons so that the total number of electrons gained by one ion is equal to the total number of electrons lost by the other ion. The two half reac,ons are: Cu0 – 2 e– and 2 Ag+ + 2 e– Cu2+ 2 Ag0 Voltaic Cells Cu0 – 2 e– Cu2+ and: 2 Ag+ + 2 e– 2 Ag0 We can see from the half equa)ons that electrons are being transferred from the Cu0 atoms to the Ag+ ions. If we can somehow put a wire between the Cu0 atoms and the Ag+ ions, we could generate an electric current between them and use this electric current to do some form of work. This is the basis of a voltaic cell (or galvanic cell). In a voltaic cell, we physically separate the two half reac)ons and connect them via an electrical circuit. This Cu/Ag cell generates a poten)al difference of 0.462 V. Salt Bridges Note the func)on of the salt bridge. If we have no salt bridge, the Cu half cell anode will start to lose electrons and generate Cu2+ ca)ons but it will not have enough nega)ve counter ions. The Cu anode will then develop a posi)ve charge that will pull the electrons back. Likewise, the Ag half-‐cell cathode will start to gain electrons and reduce Ag+ ca)ons to Ag0 metal but it will then have too many nega)ve counter ions. This will result in a nega)ve charge that will repel the electric current. In short, the current will soon stop flowing. By using a salt bridge, however, NO3– counter ions can cross from the Ag cathode to the Cu anode and balance the charges. The salt bridge therefore creates a closed circuit involving the Cu anode, the electrical wires, the Ag cathode and the salt bridge itself. In this way, counter ions travel through the salt bridge and electrons travel through the wires. Ba[eries Voltaic cells are the basis of ba[eries. For example, a Zn-‐Cu cell generates a poten)al difference of 1.100 volts (V). By connec)ng voltaic cells in series, however, we can add the poten)al differences together to make a ba[ery with double the poten)al difference. Note that, if we connect the cells in parallel, the poten)al difference will remain at 1.100V but double the current can flow before the cells run out of one of the metals. The Hydrogen Fuel Cell An important kind of cell for the 21st century is the fuel cell. These cells use hydrogen and oxygen for the redox reac)on and produce water as the waste product. In this way, we can generate electricity from the reac)on between hydrogen and oxygen to produce water. This is an important step in reducing our need for fossil fuels that cause climate change. Current research lies in developing catalysts that accelerate the redox processes at the electrodes and in developing more efficient polymer electrolytes that allow protons to flow from the H2 anode to the O2 cathode. Solar Powered Electrolysis Currently, hydrogen is produced mainly from methane gas (CH4) and coal so we are s)ll dependent on fossil fuels as the ul)mate source of our hydrogen power. However, if we can use solar power to split water into hydrogen and oxygen gas, we can ul)mately power civilisa)on with the Sun. By developing new catalysts that harness the energy of sunlight to split water, we can make solar powered water electrolysis cells. Corrosion Corrosion is a process where materials are gradually destroyed by chemical reac)ons. The most common form of corrosion is the oxida)on of metals by oxygen — we are especially familiar with the case of rus)ng iron (or steel), for example. Since oxida)on of metals is a redox process, we can use the study of electrochemistry to be[er understand, and thereby prevent, corrosion. Let’s take the case of iron. The first stage of rus)ng is caused by the spontaneous forma)on of Fe2+ ions at points of high strain. Since this is a point of oxida)on, it is called the anodic region. The electrons released from these ions can travel through the metal and cataly)cally reduce oxygen atoms to hydroxide ions in the presence of water. Since oxygen is being reduced, this point is called the cathodic region. 1) Fe —> Fe2+ + 2e– (oxida)on) 2) O2 + 2H2O + 4e– —> 4OH– (reduc)on) Overall: 2Fe + O2 + 2H2O —> 2Fe2+ + 4OH– The Fe2+ ions can travel through moisture on the surface of the iron to combine with the OH– ions. This migra)on balances the charges and allows more Fe2+ and OH– to form. Thereaber, the Fe2+ ions are oxidised by more oxygen to form Fe3+ ions and make the complex mixture of Fe2O3 and water known as rust. 2Fe + 3/2O2 + xH20 —> 2Fe2O3・xH2O Rus)ng Crucially, rust cracks and breaks up spontaneously, which allows more oxygen and water to contact fresh iron, thus allowing the rus)ng process to con)nue un)l there is no more iron, oxygen or water. Corrosion Protec)on The most common way to prevent corrosion is to simply paint the surface. If no oxygen and moisture can reach the surface, the metal cannot be oxidised. Another common method is to use electropla)ng to plate the surface of the metal with a metal that is less easily corroded. The thin layer of )n on )n cans protects the steel underneath. Sacrificial Anodes The problem with paint and )n pla)ng is that they cannot protect the surface aber damage exposes the underlying metal. However, chemists have been able to make use of electrochemistry to prevent corrosion of even cracked surfaces. Remember that electrons flow from the anodic point of strain to generate Fe2+ ions. If we connect a metal that is more easily oxidised, electrons will flow from the easily oxidised metal to the point of strain and prevent the forma)on of the Fe2+ ions. Magnesium is a useful sacrificial anode for steel (Fe). Fe2+ e– Mg Galvanising (zinc pla)ng) steel implements has a double effect. Firstly, the zinc protects the surface and secondly, the zinc acts as a sacrificial anode to prevent rus)ng even if the surface is damaged. Corrosion Resistant Metals Note that not all metals corrode. Aluminium is so reac)ve that it quickly forms an oxide layer on its surface. However, unlike rust, aluminium oxide does not crack and peel and so it automa)cally forms a protec)ve surface. For this reason, aluminium surfaces are not usually very shiny but we do not have to worry if they get scratched — a new, protec)ve oxide layer will quickly form again. Reading and Problems Reading Chapter 21: 21-‐8, 21-‐9, 21-‐10, (21-‐22), 21-‐17, 21-‐18, 21 -‐25, Problems Chapter 21: 15, 36, 44,