Chapter 13
... chemical change in a reaction in an aq soln. – To create an ionic equation, must convert a balanced chemical equation into an ionic eq. ...
... chemical change in a reaction in an aq soln. – To create an ionic equation, must convert a balanced chemical equation into an ionic eq. ...
Ionization Spectroscopy of a DNA Base: Vacuum
... associated with the DNA base ionization and future molecular electronic devices. In the present study, we used mass-analyzed threshold ionization (MATI) spectroscopy to determine the ionization potential and cationic vibrational structure of the DNA base, thymine. Recent extensive studies on DNA bas ...
... associated with the DNA base ionization and future molecular electronic devices. In the present study, we used mass-analyzed threshold ionization (MATI) spectroscopy to determine the ionization potential and cationic vibrational structure of the DNA base, thymine. Recent extensive studies on DNA bas ...
The Relation between Salt and Ionic Transport Coefficients
... the whole salt moving as a single electrically neutral component or for the individual ions of the salt. The latter definition is meaningful only in the absence of an electric field across the permeability barrier. This condition may be achieved with the voltage clamp or short-circuit technique and ...
... the whole salt moving as a single electrically neutral component or for the individual ions of the salt. The latter definition is meaningful only in the absence of an electric field across the permeability barrier. This condition may be achieved with the voltage clamp or short-circuit technique and ...
Coulomb effect in multiphoton ionization of rare
... rates of these two theories agree as will be shown (Perelomov, Popov and Terent’ev’s model (1966) will be referred to as the PPT model). Other approaches for including the Coulomb field have also been proposed which successfully explain some aspects of the ATI ionization (Kamiński and Ehlotzky 1997 ...
... rates of these two theories agree as will be shown (Perelomov, Popov and Terent’ev’s model (1966) will be referred to as the PPT model). Other approaches for including the Coulomb field have also been proposed which successfully explain some aspects of the ATI ionization (Kamiński and Ehlotzky 1997 ...
Detection of Organic Pollutants with a Pulsed Ion Mobility
... Ion Mobility Spectrometry is a standard technique used to analyze ambient air [1]. The advantages are a small detector size (typically consisiting of a tube which is ca. 8 cm long with a diameter of ca. 2 cm), a high sensitivity in the ppb range and a fast response time of a few seconds. The informa ...
... Ion Mobility Spectrometry is a standard technique used to analyze ambient air [1]. The advantages are a small detector size (typically consisiting of a tube which is ca. 8 cm long with a diameter of ca. 2 cm), a high sensitivity in the ppb range and a fast response time of a few seconds. The informa ...
Honors Review for Semester 1 Final 2014
... 2. You should also be able to illustrate the above scientists’ models of the atoms, labeling subatomic particles. 3. Define atom, nucleus, proton, neutrons, and electron. 4. From the atomic number and the mass number, you should be able to give the name of the element, number of protons, neutrons, a ...
... 2. You should also be able to illustrate the above scientists’ models of the atoms, labeling subatomic particles. 3. Define atom, nucleus, proton, neutrons, and electron. 4. From the atomic number and the mass number, you should be able to give the name of the element, number of protons, neutrons, a ...
2.3 Atomic and Molecular Collisions
... negatively and positively charged molecules and clusters [1]. The beams for CSR were produced in its ion source area from discharge and sputter ion sources. Aside from its electronic diagnostic elements for stored ions, the CSR was equipped with detectors [2] for the fast particles produced when sto ...
... negatively and positively charged molecules and clusters [1]. The beams for CSR were produced in its ion source area from discharge and sputter ion sources. Aside from its electronic diagnostic elements for stored ions, the CSR was equipped with detectors [2] for the fast particles produced when sto ...
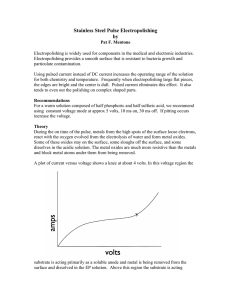
This is an overview of what can happen during the
... primarily as an insoluble anode and the water is being electrolyzed to oxygen gas and hydrogen ions. In order for polishing to occur, the current and voltage must be above the knee of the curve, this location is marked with an X in the above graph If the substrate functioned as a 100% soluble anode ...
... primarily as an insoluble anode and the water is being electrolyzed to oxygen gas and hydrogen ions. In order for polishing to occur, the current and voltage must be above the knee of the curve, this location is marked with an X in the above graph If the substrate functioned as a 100% soluble anode ...
Exercised Review for Test
... 22. In the N2 molecule, what is the number of unshared pairs of electrons in each nitrogen atom? a. 1 ...
... 22. In the N2 molecule, what is the number of unshared pairs of electrons in each nitrogen atom? a. 1 ...
Unit 1 - Learning Objectives
... The noble gases are a family of very unreactive elements. The alkali metals are a family of very reactive metals. The halogens are a family of very reactive non-metals. The transition metals are found between Groups 2 and 3 in the Periodic Table. (ii) Compounds and mixtures Most compounds ...
... The noble gases are a family of very unreactive elements. The alkali metals are a family of very reactive metals. The halogens are a family of very reactive non-metals. The transition metals are found between Groups 2 and 3 in the Periodic Table. (ii) Compounds and mixtures Most compounds ...
Are diglycolamide ligands hard or soft Lewis bases?
... The energy and Gibbs free energy of formation of the [M(TEDGA)3]3+ complexes are less negative than the respective values of the [M(BTBP)2]3+ complexes, but the differences are not so big, less than 10 kJ/mol. ...
... The energy and Gibbs free energy of formation of the [M(TEDGA)3]3+ complexes are less negative than the respective values of the [M(BTBP)2]3+ complexes, but the differences are not so big, less than 10 kJ/mol. ...
Slide 1
... electrons are shared by two atoms. • Ionic bond: one or more electrons from one atom are removed and attached to another atom. • resulting in positive and negative ions which attract each other. ...
... electrons are shared by two atoms. • Ionic bond: one or more electrons from one atom are removed and attached to another atom. • resulting in positive and negative ions which attract each other. ...
JCA 2007 (vol 1159, pp 51-57)
... large number of background signals, including sodium clusters. The mebeverine signal using the anionic CD is even more reduced compared to using the neutral CD, which could be due to the fact that ionization suppression is caused by both the CD and its sodium counter ions. However, using HDMS-β-CD t ...
... large number of background signals, including sodium clusters. The mebeverine signal using the anionic CD is even more reduced compared to using the neutral CD, which could be due to the fact that ionization suppression is caused by both the CD and its sodium counter ions. However, using HDMS-β-CD t ...
Matter-Wave Metrology as a Complementary Tool for Mass
... under identical ionization conditions and spectrometer settings as used for the initial experiments. As this molecule is a structural isomer of the fragment 2, it showed the same interference curves and power dependence as observed in the initial experiments with 1 as source material, as expected. W ...
... under identical ionization conditions and spectrometer settings as used for the initial experiments. As this molecule is a structural isomer of the fragment 2, it showed the same interference curves and power dependence as observed in the initial experiments with 1 as source material, as expected. W ...
Degrees of freedom effect on fragmentation in tandem mass
... share the same CCE. A similar trend with a somewhat lower slope is observed for negatively charged aggregates (Fig. 3). The present results on clusters may be compared with the behavior of PEGs shown in Fig. 4. This shows data on PEGs both measured in this study (triangles) and taken from the litera ...
... share the same CCE. A similar trend with a somewhat lower slope is observed for negatively charged aggregates (Fig. 3). The present results on clusters may be compared with the behavior of PEGs shown in Fig. 4. This shows data on PEGs both measured in this study (triangles) and taken from the litera ...
CHEMISTRY 11 Unit 4 Assignment - The Mole
... 13. How much mass and volume (at STP) of gaseous tetraphosphorus hexaoxide would be present if 9.63 x 1024 atoms of oxygen were found in the sample? (4) ...
... 13. How much mass and volume (at STP) of gaseous tetraphosphorus hexaoxide would be present if 9.63 x 1024 atoms of oxygen were found in the sample? (4) ...
I believe the chemical bond is not so simple as people seem to think
... Its existence was for a long delicate molecule formed by two helium atoms requires a light touch. time disputed because of its extremely small binding enThe helium dimer is the largest two-atom molecule and has the ...
... Its existence was for a long delicate molecule formed by two helium atoms requires a light touch. time disputed because of its extremely small binding enThe helium dimer is the largest two-atom molecule and has the ...
I 14-7 ION CHEMISTRY
... into molecular ions with a range of internal energy states (Section 13-3). In some collisions the minimum energy required to cause ionization, the ionization energy IE, is transferred to the molecule. This situation leads to formation of the ion AB+· in its ground state, with zero internal energy. I ...
... into molecular ions with a range of internal energy states (Section 13-3). In some collisions the minimum energy required to cause ionization, the ionization energy IE, is transferred to the molecule. This situation leads to formation of the ion AB+· in its ground state, with zero internal energy. I ...
S90 Notes U2 Topic 6 Chemical Compounds
... S90 Notes U2 Topic 6 Chemical Compounds Chemical compounds are formed by 2 or more elements. There are 2 types of compounds – ionic compounds and molecular compounds ...
... S90 Notes U2 Topic 6 Chemical Compounds Chemical compounds are formed by 2 or more elements. There are 2 types of compounds – ionic compounds and molecular compounds ...
Chemical Bonding Notes for 2016
... • Metals do not combine with metals. • They form alloys which is a solution of a metal in a metal. • Examples are steel, brass, bronze and pewter. ...
... • Metals do not combine with metals. • They form alloys which is a solution of a metal in a metal. • Examples are steel, brass, bronze and pewter. ...
+1/2 and
... Electron affinity (A) is the energy gain that we get if an electron is absorbed by an atom or a molecule. Relation to ionization energy, e.g. oxygen: IO AO Electron affinities of some atoms (kJ mol-1) ...
... Electron affinity (A) is the energy gain that we get if an electron is absorbed by an atom or a molecule. Relation to ionization energy, e.g. oxygen: IO AO Electron affinities of some atoms (kJ mol-1) ...
Working with solutions
... O A base is any substance that forms hydroxide ions in water. O A strong acid will produce more hydrogen ions than a weak one. O A strong base will produce more hydroxide ions than a weak one. ...
... O A base is any substance that forms hydroxide ions in water. O A strong acid will produce more hydrogen ions than a weak one. O A strong base will produce more hydroxide ions than a weak one. ...
Word - chemmybear.com
... There are three types of bonds, but four types of solids held together with these bonds. Lattice: a repeating pattern, like a lattice-work fence. In solids, it is a repeating pattern of atoms. All solids are made up of a lattice. The points of the lattice are different in different types of solids. ...
... There are three types of bonds, but four types of solids held together with these bonds. Lattice: a repeating pattern, like a lattice-work fence. In solids, it is a repeating pattern of atoms. All solids are made up of a lattice. The points of the lattice are different in different types of solids. ...
BH - hrsbstaff.ednet.ns.ca
... to a known amount of another substance. An indicator is most commonly used to signal when a reaction is complete. Endpoint- the volume of the added reactant (titrant) required to complete the reaction. ...
... to a known amount of another substance. An indicator is most commonly used to signal when a reaction is complete. Endpoint- the volume of the added reactant (titrant) required to complete the reaction. ...
Ion source

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.