* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Bonding Notes for 2016
Stability constants of complexes wikipedia , lookup
Electron scattering wikipedia , lookup
State of matter wikipedia , lookup
Photoelectric effect wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Degenerate matter wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Aromaticity wikipedia , lookup
Ionic liquid wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Electrochemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Ionic compound wikipedia , lookup
Electron configuration wikipedia , lookup
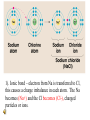
Why are electrons important? • Chemical bonding involves electrons in the outermost energy level (valence electrons) . • When chemical bonds are formed, atoms gain, lose or share electrons to have 8 electrons in their valence level. • Exception: He (duet rule) C would like to N would like to O would like to Gain 4 electrons Gain 3 electrons Gain 2 electrons Chemical bonds • Atoms attempt to fill electron shells. • When electrons are gained, lost or shared, an attractive force is formed called a BOND, which holds elements together. -Ionic bonds (metal & non metal) -Covalent bonds (2 non metals) -Metallic bonds (2 metals) Ionic Bonding • Occurs between a metal and a non-metal. • Electrons are moved from one atom to another. • The charged particle that results is called an ION. IONS • When an atom loses (or gives away) its electrons, it becomes a positively charged. • This is known as a CATION. • Metals commonly form positive ions. IONS Continued.. • When an atom gains electrons, it becomes negatively charged. • This is known as an ANION. • Non metals commonly form negative ions. Formation of Ions from Metals Positive ions form when the number of electrons are less than the number of protons Group 1 metals ion 1+ Group 2 metals ion 2+ Group 13 metals ion 3+ Formation of Sodium Ion Sodium atom Na 2-8-1 11 p+ 11 e0 – e Sodium ion Na + 2-8 ( = Ne) 11 p+ 10 e1+ Formation of Magnesium Ion Magnesium atom Magnesium ion Mg 2-8-2 12 p+ 12 e0 – 2e Mg2+ 2-8 (=Ne) 12 p+ 10 e2+ Typical Ions with Positive Charges (Cations) Group 1 Group 2 Group 13 H+ Mg2+ Al3+ Li+ Ca2+ Na+ Sr2+ K+ Ba2+ Learning Check A. Number of valence electrons in aluminum 1) 1 e2) 2 e3) 3 eB. C. Change in electrons for octet 1) lose 3e2) gain 3 eIonic charge of aluminum 1) 32) 5- 3) gain 5 e- 3) 3+ Solution A. Number of valence electrons in aluminum 3) 3 eB. Change in electrons for octet 1) lose 3e- C. Ionic charge of aluminum 3) 3+ Formation of Ions from Nonmetals • In ionic compounds, nonmetals in 15, 16, and 17 gain electrons from metals • Nonmetals gain electrons to achieve a stable octet • Nonmetal ionic charge: -3, -2, or -1 Fluoride Ion unpaired electron :F 2-7 9 p+ 9 e0 + e octet 1- : F: 2-8 (= Ne) 9 p+ 10 e-1 ionic charge Ionic Bond Properties • Crystal lattice: repeating 3D pattern Ionic Bond Properties • Strong attraction between ions result in brittleness. Ionic Bond Properties • High melting points & usually solid at room temperature. Ionic Bond Properties • Soluble • Good Conductors Ionic Bonds: One Big Greedy Thief Dog! http://web.jjay.cuny.edu/~acarpi/NSC/salt.htm 1). Ionic bond – electron from Na is transferred to Cl, this causes a charge imbalance in each atom. The Na becomes (Na+) and the Cl becomes (Cl-), charged particles or ions. Oxidation Numbers • The number of electrons that an element can lose, gain or share is called the OXIDATION NUMBER. • Some elements have more than one oxidation number, example Fe +2 or Fe+3 Formula Weights Or Molecular mass • Formula weight is the sum of the atomic masses. • Example- CO2 • Mass, C + O + O 12.011 + 15.994 + 15.994 43.999 Covalent Bond • Between 2 or more nonmetal elements. • Formed by sharing electron pairs. • Smallest unit of a covalently bonded compound is called: molecule • Examples; O2, CO2, C2H6, SiC Covalent Bond Properties • Low melting point & boiling point • They are not conductors at any state. . POLAR COVALENT BONDS Electrons are shared but shared unequally H2O Polar Covalent Bonds Unevenly matched, but willing to share polyatomic ions A covalently bonded group of atoms acting as one that have a positive or negative charge. Diatomic Molecules • Some elements exist in nature as covalent bonds. • Composed of only 2 elements Diatomic Molecules • Nonmetals called the “Heavenly 7” Heavenly 7 Mnemonic • HONClBrIF The Chemistry Goose • Pronounced “Honk-Cool-Brief” Metallic Bond • Formed between atoms of metallic elements • Metal atoms become cations surrounded by a pool of electrons • Good conductors at all states, lustrous, very high melting points • Examples; Na, Fe, Al, Au, Co Metallic Bonds: Mellow dogs with plenty of bones to go around. Metallic Bond Attraction between a metal cation and shared electrons. Metals Form Alloys • Metals do not combine with metals. • They form alloys which is a solution of a metal in a metal. • Examples are steel, brass, bronze and pewter. Practice • Compute the mass of the following compounds round to nearest tenth & state type of bond: • NaCl; • 23 + 35 = 58; Ionic Bond • C2H6; • 24 + 6 = 30; Covalent Bond • Na(CO3)2; • 23 + 2(12 + 3x16) = 123; Ionic & Covalent The End